Smooth muscle cell calcium activation mechanisms
- PMID: 18787034
- PMCID: PMC2652144
- DOI: 10.1113/jphysiol.2008.160440
Smooth muscle cell calcium activation mechanisms
Abstract
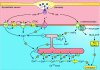
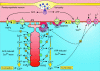
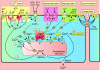
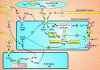
Smooth muscle cell (SMC) contraction is controlled by the Ca2+ and Rho kinase signalling pathways. While the SMC Rho kinase system seems to be reasonably constant, there is enormous variation with regard to the mechanisms responsible for generating Ca2+ signals. One way of dealing with this diversity is to consider how this system has been adapted to control different SMC functions. Phasic SMCs (vas deferens, uterus and bladder) rely on membrane depolarization to drive Ca2+ influx across the plasma membrane. This depolarization can be induced by neurotransmitters or through the operation of a membrane oscillator. Many tonic SMCs (vascular, airway and corpus cavernosum) are driven by a cytosolic Ca2+ oscillator that generates periodic pulses of Ca2+. A similar oscillator is present in pacemaker cells such as the interstitial cells of Cajal (ICCs) and atypical SMCs that control other tonic SMCs (gastrointestinal, urethra, ureter). The changes in membrane potential induced by these cytosolic oscillators does not drive contraction directly but it functions to couple together individual oscillators to provide the synchronization that is a characteristic feature of many tonic SMCs.
Figures



References
-
- Aoyama M, Yamada A, Wang J, Ohya S, Furuzono S, Goto T, Hotta S, Ito Y, Matsubara T, Shimokata K, Chen SRW, Imaizumi Y, Nakayama S. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sci. 2004;117:2813–2825. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium oscillations. Biochem Soc Symp. 2007;74:1–7. - PubMed
-
- Berridge MJ. Cell Signalling Biology. Portland Press Limited; 2008. http://www.cellsignallingbiology.org.
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

