Axl and Tyro3 modulate female reproduction by influencing gonadotropin-releasing hormone neuron survival and migration
- PMID: 18787040
- PMCID: PMC2582545
- DOI: 10.1210/me.2008-0169
Axl and Tyro3 modulate female reproduction by influencing gonadotropin-releasing hormone neuron survival and migration
Abstract
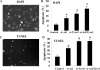
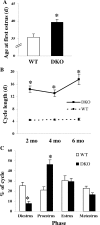
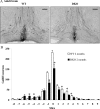
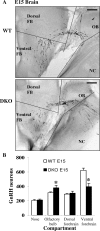
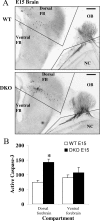
GnRH neurons must undergo a complex and precise pattern of neuronal migration to appropriately target their projections to the median eminence to trigger gonadotropin secretion and thereby control reproduction. Using NLT GnRH cells as a model of early GnRH neuronal development, we identified the potential importance of Axl and Tyro3, members of the TAM (Tyro3, Axl, and Mer) family of receptor tyrosine kinases in GnRH neuronal cell survival and migration. Silencing studies evaluated the role of Tyro3 and Axl in NLT GnRH neuronal cells and suggest that both play a role in Gas6 stimulation of GnRH neuronal survival and migration. Analysis of mice null for both Axl and Tyro3 showed normal onset of vaginal opening but delayed first estrus and persistently abnormal estrous cyclicity compared with wild-type controls. Analysis of GnRH neuronal numbers and positioning in the adult revealed a total loss of 24% of the neuronal network that was more striking (34%) when considered within specific anatomical compartments, with the largest deficit surrounding the organum vasculosum of the lamina terminalis. Analysis of GnRH neurons during embryogenesis identified a striking loss of immunoreactive cells within the context of the ventral forebrain compartment (36%) and not more rostrally. Studies using caspase 3 cleavage as a marker of apoptosis showed that Axl(-/-), Tyro3(-/-) double-knockout mice had increased cell death in the nose and dorsal forebrain, supporting the underlying mechanism of cell loss. Together these data suggest that Axl and Tyro3 mediate the survival and appropriate targeting of GnRH neurons to the ventral forebrain, thereby contributing to normal reproductive function and cyclicity in the female.
Figures

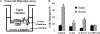

References
-
- Wray S, Hoffman G 1986 Postnatal morphological changes in rat LHRH neurons correlated with sexual maturation. Neuroendocrinology 43:93–97 - PubMed
-
- Schwanzel-Fukuda M, Bick D, Pfaff DW 1989 Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res 6:311–326 - PubMed
-
- Tobet SA, Schwarting GA 2006 Recent progress in gonadotropin-releasing hormone neuronal migration. Endocrinology 147:1159–1165 - PubMed
-
- Schwarting GA, Wierman ME, Tobet SA 2007 Gonadotropin-releasing hormone neuronal migration. Semin Reprod Med 25:305–312 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

