odd skipped related1 reveals a novel role for endoderm in regulating kidney versus vascular cell fate
- PMID: 18787069
- PMCID: PMC2742377
- DOI: 10.1242/dev.022830
odd skipped related1 reveals a novel role for endoderm in regulating kidney versus vascular cell fate
Abstract
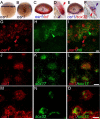
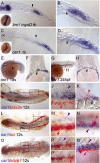
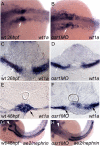
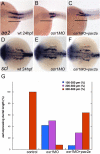
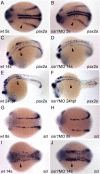
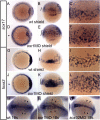
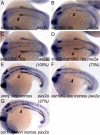
The kidney and vasculature are intimately linked both functionally and during development, when nephric and blood/vascular progenitor cells occupy adjacent bands of mesoderm in zebrafish and frog embryos. Developmental mechanisms that underlie the differentiation of kidney versus blood/vascular lineages remain unknown. The odd skipped related1 (osr1) gene encodes a zinc-finger transcription factor that is expressed in the germ ring mesendoderm and subsequently in the endoderm and intermediate mesoderm, prior to the expression of definitive kidney or blood/vascular markers. Knockdown of osr1 in zebrafish embryos resulted in a complete, segment-specific loss of anterior kidney progenitors and a compensatory increase in the number of angioblast cells in the same trunk region. Histology revealed a subsequent absence of kidney tubules, an enlarged cardinal vein and expansion of the posterior venous plexus. Altered kidney versus vascular development correlated with expanded endoderm development in osr1 knockdowns. Combined osr1 loss of function and blockade of endoderm development by knockdown of sox32/casanova rescued anterior kidney development. The results indicate that osr1 activity is required to limit endoderm differentiation from mesendoderm; in the absence of osr1, excess endoderm alters mesoderm differentiation, shifting the balance from kidney towards vascular development.
Figures




Similar articles
-
Odd skipped related 1 is a negative feedback regulator of nodal-induced endoderm development.Dev Dyn. 2014 Dec;243(12):1571-80. doi: 10.1002/dvdy.24191. Epub 2014 Oct 18. Dev Dyn. 2014. PMID: 25233796 Free PMC article.
-
osr1 is required for podocyte development downstream of wt1a.J Am Soc Nephrol. 2014 Nov;25(11):2539-45. doi: 10.1681/ASN.2013121327. Epub 2014 Apr 10. J Am Soc Nephrol. 2014. PMID: 24722440 Free PMC article.
-
Molecular integration of casanova in the Nodal signalling pathway controlling endoderm formation.Development. 2002 Jan;129(2):275-86. doi: 10.1242/dev.129.2.275. Development. 2002. PMID: 11807021
-
From Endoderm to Progenitors: An Update on the Early Steps of Thyroid Morphogenesis in the Zebrafish.Front Endocrinol (Lausanne). 2021 Jun 4;12:664557. doi: 10.3389/fendo.2021.664557. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34149617 Free PMC article. Review.
-
Patterning of the zebrafish embryo by nodal signals.Curr Top Dev Biol. 2003;55:143-71. doi: 10.1016/s0070-2153(03)01003-2. Curr Top Dev Biol. 2003. PMID: 12959195 Review. No abstract available.
Cited by
-
Molecular regulation of kidney development.Anat Cell Biol. 2013 Mar;46(1):19-31. doi: 10.5115/acb.2013.46.1.19. Epub 2013 Mar 25. Anat Cell Biol. 2013. PMID: 23560233 Free PMC article.
-
osr1 Maintains Renal Progenitors and Regulates Podocyte Development by Promoting wnt2ba via the Antagonism of hand2.Biomedicines. 2022 Nov 9;10(11):2868. doi: 10.3390/biomedicines10112868. Biomedicines. 2022. PMID: 36359386 Free PMC article.
-
Eya-six are necessary for survival of nephrogenic cord progenitors and inducing nephric duct development before ureteric bud formation.Dev Dyn. 2015 Jul;244(7):866-73. doi: 10.1002/dvdy.24282. Epub 2015 Jun 18. Dev Dyn. 2015. PMID: 25903664 Free PMC article.
-
Odd skipped related 1 is a negative feedback regulator of nodal-induced endoderm development.Dev Dyn. 2014 Dec;243(12):1571-80. doi: 10.1002/dvdy.24191. Epub 2014 Oct 18. Dev Dyn. 2014. PMID: 25233796 Free PMC article.
-
The opening act: vasculogenesis and the origins of circulation.Arterioscler Thromb Vasc Biol. 2009 May;29(5):623-9. doi: 10.1161/ATVBAHA.107.161539. Epub 2008 Nov 13. Arterioscler Thromb Vasc Biol. 2009. PMID: 19008532 Free PMC article. Review.
References
-
- Alexander J, Rothenberg M, Henry GL, Stainier DY. casanova plays an early and essential role in endoderm formation in zebrafish. Dev Biol. 1999;215:343–57. - PubMed
-
- Barrallo-Gimeno A, Holzschuh J, Driever W, Knapik EW. Neural crest survival and differentiation in zebrafish depends on mont blanc/tfap2a gene function. Development. 2004;131:1463–77. - PubMed
-
- Bisgrove BW, Raible DW, Walter V, Eisen JS, Grunwald DJ. Expression of c-ret in the zebrafish embryo: potential roles in motoneuronal development. J Neurobiol. 1997;33:749–68. - PubMed
-
- Bollig F, Mehringer R, Perner B, Hartung C, Schafer M, Schartl M, Volff JN, Winkler C, Englert C. Identification and comparative expression analysis of a second wt1 gene in zebrafish. Dev Dyn. 2006;235:554–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

