Oxidant-induced inhibition of the plasma membrane Ca2+-ATPase in pancreatic acinar cells: role of the mitochondria
- PMID: 18787078
- PMCID: PMC2584981
- DOI: 10.1152/ajpcell.00083.2008
Oxidant-induced inhibition of the plasma membrane Ca2+-ATPase in pancreatic acinar cells: role of the mitochondria
Abstract
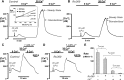
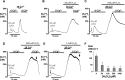
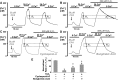
Impairment of the normal spatiotemporal pattern of intracellular Ca(2+) ([Ca(2+)](i)) signaling, and in particular, the transition to an irreversible "Ca(2+) overload" response, has been implicated in various pathophysiological states. In some diseases, including pancreatitis, oxidative stress has been suggested to mediate this Ca(2+) overload and the associated cell injury. We have previously demonstrated that oxidative stress with hydrogen peroxide (H(2)O(2)) evokes a Ca(2+) overload response and inhibition of plasma membrane Ca(2+)-ATPase (PMCA) in rat pancreatic acinar cells (Bruce JI and Elliott AC. Am J Physiol Cell Physiol 293: C938-C950, 2007). The aim of the present study was to further examine this oxidant-impaired inhibition of the PMCA, focusing on the role of the mitochondria. Using a [Ca(2+)](i) clearance assay in which mitochondrial Ca(2+) uptake was blocked with Ru-360, H(2)O(2) (50 microM-1 mM) markedly inhibited the PMCA activity. This H(2)O(2)-induced inhibition of the PMCA correlated with mitochondrial depolarization (assessed using tetramethylrhodamine methylester fluorescence) but could occur without significant ATP depletion (assessed using Magnesium Green fluorescence). The H(2)O(2)-induced PMCA inhibition was sensitive to the mitochondrial permeability transition pore (mPTP) inhibitors, cyclosporin-A and bongkrekic acid. These data suggest that oxidant-induced opening of the mPTP and mitochondrial depolarization may lead to an inhibition of the PMCA that is independent of mitochondrial Ca(2+) handling and ATP depletion, and we speculate that this may involve the release of a mitochondrial factor. Such a phenomenon may be responsible for the Ca(2+) overload response, and for the transition between apoptotic and necrotic cell death thought to be important in many disease states.
Figures



Similar articles
-
Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries.J Physiol. 2004 May 1;556(Pt 3):755-71. doi: 10.1113/jphysiol.2003.059568. Epub 2004 Feb 6. J Physiol. 2004. PMID: 14766935 Free PMC article.
-
Plasma membrane Ca2+-ATPase isoforms composition regulates cellular pH homeostasis in differentiating PC12 cells in a manner dependent on cytosolic Ca2+ elevations.PLoS One. 2014 Jul 11;9(7):e102352. doi: 10.1371/journal.pone.0102352. eCollection 2014. PLoS One. 2014. PMID: 25014339 Free PMC article.
-
Oxidant-impaired intracellular Ca2+ signaling in pancreatic acinar cells: role of the plasma membrane Ca2+-ATPase.Am J Physiol Cell Physiol. 2007 Sep;293(3):C938-50. doi: 10.1152/ajpcell.00582.2006. Epub 2007 May 9. Am J Physiol Cell Physiol. 2007. PMID: 17494627
-
Mitochondrial function and malfunction in the pathophysiology of pancreatitis.Pflugers Arch. 2012 Jul;464(1):89-99. doi: 10.1007/s00424-012-1117-8. Epub 2012 Jun 1. Pflugers Arch. 2012. PMID: 22653502 Review.
-
Metabolic regulation of the PMCA: Role in cell death and survival.Cell Calcium. 2018 Jan;69:28-36. doi: 10.1016/j.ceca.2017.06.001. Epub 2017 Jun 8. Cell Calcium. 2018. PMID: 28625348 Free PMC article. Review.
Cited by
-
Ethanol and its non-oxidative metabolites profoundly inhibit CFTR function in pancreatic epithelial cells which is prevented by ATP supplementation.Pflugers Arch. 2014 Mar;466(3):549-62. doi: 10.1007/s00424-013-1333-x. Epub 2013 Aug 16. Pflugers Arch. 2014. PMID: 23948742
-
Novel lipophilic probe for detecting near-membrane reactive oxygen species responses and its application for studies of pancreatic acinar cells: effects of pyocyanin and L-ornithine.Antioxid Redox Signal. 2015 Feb 20;22(6):451-64. doi: 10.1089/ars.2013.5589. Epub 2014 Apr 30. Antioxid Redox Signal. 2015. PMID: 24635199 Free PMC article.
-
Redox signaling in acute pancreatitis.Redox Biol. 2015 Aug;5:1-14. doi: 10.1016/j.redox.2015.01.014. Epub 2015 Jan 28. Redox Biol. 2015. PMID: 25778551 Free PMC article. Review.
-
Insulin protects acinar cells during pancreatitis by preserving glycolytic ATP supply to calcium pumps.Nat Commun. 2021 Jul 19;12(1):4386. doi: 10.1038/s41467-021-24506-w. Nat Commun. 2021. PMID: 34282152 Free PMC article.
-
Peripheral biomarkers of oxidative stress in dogs with acute pancreatitis.J Vet Intern Med. 2022 Nov;36(6):1958-1965. doi: 10.1111/jvim.16535. Epub 2022 Sep 10. J Vet Intern Med. 2022. PMID: 36086902 Free PMC article.
References
-
- Baggaley E, McLarnon S, Demeter I, Varga G, Bruce JI. Differential regulation of the apical plasma membrane Ca2+-ATPase by protein kinase A in parotid acinar cells. J Biol Chem 282: 37678–37693, 2007. - PubMed
-
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005. - PubMed
-
- Barros LF, Kanaseki T, Sabirov R, Morishima S, Castro J, Bittner CX, Maeno E, Ando-Akatsuka Y, Okada Y. Apoptotic and necrotic blebs in epithelial cells display similar neck diameters but different kinase dependency. Cell Death Differ 10: 687–697, 2003. - PubMed
-
- Barrow SL, Voronina SG, da Silva Xavier G, Chvanov MA, Longbottom RE, Gerasimenko OV, Petersen OH, Rutter GA, Tepikin AV. ATP depletion inhibits Ca2+ release, influx and extrusion in pancreatic acinar cells but not pathological Ca2+ responses induced by bile. Pflügers Arch 455: 1025–1039, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

