Gastrointestinal dysmotility in mitochondrial neurogastrointestinal encephalomyopathy is caused by mitochondrial DNA depletion
- PMID: 18787099
- PMCID: PMC2543079
- DOI: 10.2353/ajpath.2008.080252
Gastrointestinal dysmotility in mitochondrial neurogastrointestinal encephalomyopathy is caused by mitochondrial DNA depletion
Abstract
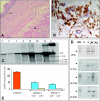
Chronic intestinal pseudo-obstruction is a life-threatening condition of unknown pathogenic mechanisms. Chronic intestinal pseudo-obstruction can be a feature of mitochondrial disorders, such as mitochondrial neurogastrointestinal encephalomyopathy (MNGIE), a rare autosomal-recessive syndrome, resulting from mutations in the thymidine phosphorylase gene. MNGIE patients show elevated circulating levels of thymidine and deoxyuridine, and accumulate somatic mitochondrial DNA (mtDNA) defects. The present study aimed to clarify the molecular basis of chronic intestinal pseudo-obstruction in MNGIE. Using laser capture microdissection, we correlated the histopathological features with mtDNA defects in different tissues from the gastrointestinal wall of five MNGIE and ten control patients. We found mtDNA depletion, mitochondrial proliferation, and smooth cell atrophy in the external layer of the muscularis propria, in the stomach and in the small intestine of MNGIE patients. In controls, the lowest amounts of mtDNA were present at the same sites, as compared with other layers of the gastrointestinal wall. We also observed mitochondrial proliferation and mtDNA depletion in small vessel endothelial and smooth muscle cells. Thus, visceral mitochondrial myopathy likely causes gastrointestinal dysmotility in MNGIE patients. The low baseline abundance of mtDNA molecules may predispose smooth muscle cells of the muscularis propria external layer to the toxic effects of thymidine and deoxyuridine, and exposure to high circulating levels of nucleosides may account for the mtDNA depletion observed in the small vessel wall.
Figures



References
-
- Connor FL, Di Lorenzo C. Chronic intestinal pseudo-obstruction: assessment and management. Gastroenterology. 2006;30:S29–S36. - PubMed
-
- Stanghellini V, Cogliandro RF, De Giorgio R, Barbara G, Salvioli B, Corinaldesi R. Chronic intestinal pseudo-obstruction: manifestations, natural history and management. Neurogastroenterol Motil. 2007;19:440–452. - PubMed
-
- Jain D, Moussa K, Tandon M, Culpepper-Morgan J, Proctor DD. Role of interstitial cells of Cajal in motility disorders of the bowel. Am J Gastroenterol. 2003;98:618–624. - PubMed
-
- Bindoff L. Mitochondrial gastroenterology. Di Mauro S, Hirano M, Schon EA, editors. Abington, UK: Informa Healthcare,; Mitochondrial Medicine. 2006:143–159.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

