Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation
- PMID: 18787100
- PMCID: PMC2543089
- DOI: 10.2353/ajpath.2008.080014
Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation
Abstract
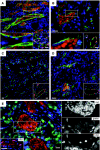
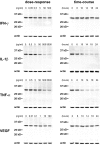
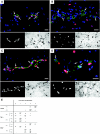
Interleukin (IL)-33 is a novel member of the IL-1 family of cytokines that promotes Th2 responses in lymphocytes as well as the activation of both mast cells and eosinophils via the ST2 receptor. Additionally, IL-33 has been proposed to act as a chromatin-associated transcriptional regulator in both endothelial cells of high endothelial venules and chronically inflamed vessels. Here we show that nuclear IL-33 is expressed in blood vessels of healthy tissues but down-regulated at the earliest onset of angiogenesis during wound healing; in addition, it is almost undetectable in human tumor vessels. Accordingly, IL-33 is induced when cultured endothelial cells reach confluence and stop proliferating but is lost when these cells begin to migrate. However, IL-33 expression was not induced by inhibiting cell cycle progression in subconfluent cultures and was not prevented by antibody-mediated inhibition of VE-cadherin. Conversely, IL-33 knockdown did not induce detectable changes in either expression levels or the cellular distribution of either VE-cadherin or CD31. However, activation of endothelial cell cultures with either tumor necrosis factor-alpha or vascular endothelial growth factor and subcutaneous injection of these cytokines led to a down-regulation of vascular IL-33, a response consistent with both its rapid down-regulation in wound healing and loss in tumor endothelium. In conclusion, we speculate that the proposed transcriptional repressor function of IL-33 may be involved in the control of endothelial cell activation.
Figures





References
-
- Onda H, Kasuya H, Takakura K, Hori T, Imaizumi T, Takeuchi T, Inoue I, Takeda J. Identification of genes differentially expressed in canine vasospastic cerebral arteries after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1999;19:1279–1288. - PubMed
-
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

