Accumulation of citrullinated proteins by up-regulated peptidylarginine deiminase 2 in brains of scrapie-infected mice: a possible role in pathogenesis
- PMID: 18787103
- PMCID: PMC2543080
- DOI: 10.2353/ajpath.2008.080388
Accumulation of citrullinated proteins by up-regulated peptidylarginine deiminase 2 in brains of scrapie-infected mice: a possible role in pathogenesis
Abstract
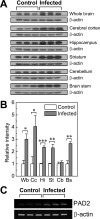
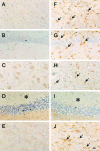
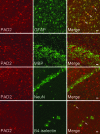
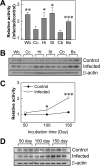
Peptidylarginine deiminases (PADs), which are a group of posttranslational modification enzymes, are involved in protein citrullination (deimination) by the conversion of peptidylarginine to peptidylcitrulline in a calcium concentration-dependent manner. Among the PADs, PAD2 is widely distributed in various tissues and is the only type that is expressed in brain. To elucidate the involvement of protein citrullination by PAD2 in the pathogenesis of brain-specific prion diseases, we examined the profiles of citrullinated proteins using the brains of scrapie-infected mice as a prion disease model. We found that, compared with controls, increased levels of citrullinated proteins of various molecular weights were detected in different brain sections of scrapie-infected mice. In support of this data, expression levels of PAD2 protein as well as its enzyme activity were significantly increased in brain sections of scrapie-infected mice, including hippocampus, brain stem, and striatum. Additionally, the expression levels of PAD2 mRNA were increased during scrapie infection. Moreover, PAD2 immunoreactivity was increased in scrapie-infected brains, with staining detected primarily in reactive astrocytes. Using two-dimensional electrophoresis and matrix-assisted laser desorption/ionization-time of flight mass spectrometry, various citrullinated proteins were identified in the brains of scrapie-infected mice, including glial fibrillary acidic protein, myelin basic protein, enolases, and aldolases. This study suggests that accumulated citrullinated proteins and abnormal activation of PAD2 may function in the pathogenesis of prion diseases and serve as potential therapeutic targets.
Figures




Similar articles
-
Abnormal accumulation of citrullinated proteins catalyzed by peptidylarginine deiminase in hippocampal extracts from patients with Alzheimer's disease.J Neurosci Res. 2005 Apr 1;80(1):120-8. doi: 10.1002/jnr.20431. J Neurosci Res. 2005. PMID: 15704193
-
Involvement of peptidylarginine deiminase-mediated post-translational citrullination in pathogenesis of sporadic Creutzfeldt-Jakob disease.Acta Neuropathol. 2010 Feb;119(2):199-210. doi: 10.1007/s00401-009-0625-x. Epub 2009 Dec 16. Acta Neuropathol. 2010. PMID: 20013286
-
Subcellular localization of peptidylarginine deiminase 2 and citrullinated proteins in brains of scrapie-infected mice: nuclear localization of PAD2 and membrane fraction-enriched citrullinated proteins.J Neuropathol Exp Neurol. 2011 Feb;70(2):116-24. doi: 10.1097/NEN.0b013e318207559e. J Neuropathol Exp Neurol. 2011. PMID: 21343880
-
Peptidylarginine deiminase and protein citrullination in prion diseases: strong evidence of neurodegeneration.Prion. 2013 Jan-Feb;7(1):42-6. doi: 10.4161/pri.22380. Epub 2012 Sep 28. Prion. 2013. PMID: 23022892 Free PMC article. Review.
-
The roles of PAD2- and PAD4-mediated protein citrullination catalysis in cancers.Int J Cancer. 2021 Jan 15;148(2):267-276. doi: 10.1002/ijc.33205. Epub 2020 Aug 8. Int J Cancer. 2021. PMID: 33459350 Review.
Cited by
-
Post-Translational Protein Deimination Signatures in Plasma and Plasma EVs of Reindeer (Rangifer tarandus).Biology (Basel). 2021 Mar 13;10(3):222. doi: 10.3390/biology10030222. Biology (Basel). 2021. PMID: 33805829 Free PMC article.
-
Enabling Global Analysis of Protein Citrullination via Biotin Thiol Tag-Assisted Mass Spectrometry.Anal Chem. 2022 Dec 27;94(51):17895-17903. doi: 10.1021/acs.analchem.2c03844. Epub 2022 Dec 13. Anal Chem. 2022. PMID: 36512406 Free PMC article.
-
Novel antiviral activity of PAD inhibitors against human beta-coronaviruses HCoV-OC43 and SARS-CoV-2.Antiviral Res. 2022 Apr;200:105278. doi: 10.1016/j.antiviral.2022.105278. Epub 2022 Mar 11. Antiviral Res. 2022. PMID: 35288208 Free PMC article.
-
Controlled Delivery of Pan-PAD-Inhibitor Cl-Amidine Using Poly(3-Hydroxybutyrate) Microspheres.Int J Mol Sci. 2021 Nov 27;22(23):12852. doi: 10.3390/ijms222312852. Int J Mol Sci. 2021. PMID: 34884657 Free PMC article.
-
PAD2 dysregulation and aberrant protein citrullination feature prominently in reactive astrogliosis and myelin protein aggregation in sporadic ALS.Neurobiol Dis. 2024 Mar;192:106414. doi: 10.1016/j.nbd.2024.106414. Epub 2024 Jan 21. Neurobiol Dis. 2024. PMID: 38253209 Free PMC article.
References
-
- Aguzzi A, Heikenwalder M, Polymenidou M. Insights into prion strains and neurotoxicity. Nat Rev Mol Cell Biol. 2007;8:552–561. - PubMed
-
- Wong K, Qiu Y, Hyun W, Nixon R, VanCleff J, Sanchez-Salazar J, Prusiner SB, DeArmond SJ. Decreased receptor-mediated calcium response in prion-infected cells correlates with decreased membrane fluidity and IP3 release. Neurology. 1996;47:741–750. - PubMed
-
- Takenouchi T, Iwamaru Y, Imamura M, Kato N, Sugama S, Fujita M, Hashimoto M, Sato M, Okada H, Yokoyama T, Mohri S, Kitani H. Prion infection correlates with hypersensitivity of P2X7 nucleotide receptor in a mouse microglial cell line. FEBS Lett. 2007;581:3019–3026. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

