Atypical E2F activity restrains APC/CCCS52A2 function obligatory for endocycle onset
- PMID: 18787127
- PMCID: PMC2567204
- DOI: 10.1073/pnas.0806510105
Atypical E2F activity restrains APC/CCCS52A2 function obligatory for endocycle onset
Abstract
The endocycle represents an alternative cell cycle that is activated in various developmental processes, including placental formation, Drosophila oogenesis, and leaf development. In endocycling cells, mitotic cell cycle exit is followed by successive doublings of the DNA content, resulting in polyploidy. The timing of endocycle onset is crucial for correct development, because polyploidization is linked with cessation of cell division and initiation of terminal differentiation. The anaphase-promoting complex/cyclosome (APC/C) activator genes CDH1, FZR, and CCS52 are known to promote endocycle onset in human, Drosophila, and Medicago species cells, respectively; however, the genetic pathways governing development-dependent APC/C(CDH1/FZR/CCS52) activity remain unknown. We report that the atypical E2F transcription factor E2Fe/DEL1 controls the expression of the CDH1/FZR orthologous CCS52A2 gene from Arabidopsis thaliana. E2Fe/DEL1 misregulation resulted in untimely CCS52A2 transcription, affecting the timing of endocycle onset. Correspondingly, ectopic CCS52A2 expression drove cells into the endocycle prematurely. Dynamic simulation illustrated that E2Fe/DEL1 accounted for the onset of the endocycle by regulating the temporal expression of CCS52A2 during the cell cycle in a development-dependent manner. Analogously, the atypical mammalian E2F7 protein was associated with the promoter of the APC/C-activating CDH1 gene, indicating that the transcriptional control of APC/C activator genes by atypical E2Fs might be evolutionarily conserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
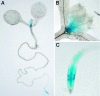

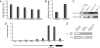
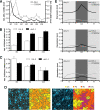
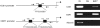
References
-
- Edgar BA, Orr-Weaver TL. Endoreplication cell cycles: More for less. Cell. 2001;105:297–306. - PubMed
-
- Nagl W. DNA endoreduplication and polyteny understood as evolutionary strategies. Science. 1976;261:614–615. - PubMed
-
- Barow M, Meister A. Endopolyploidy in seed plants is differently correlated to systematics, organ, life strategy and genome size. Plant Cell Environ. 2003;26:571–584.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

