Regulation of cytotoxic T lymphocyte triggering by PIR-B on dendritic cells
- PMID: 18787130
- PMCID: PMC2533681
- DOI: 10.1073/pnas.0804571105
Regulation of cytotoxic T lymphocyte triggering by PIR-B on dendritic cells
Abstract
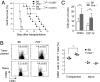
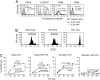
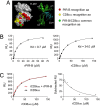
Priming of cytotoxic T lymphocytes (CTLs) by dendritic cells (DCs) is crucial for elimination of pathogens and malignant cells. To activate CTLs, DCs present antigenic peptide-complexed MHC class I molecules (MHC-I) that will be recognized by the CTLs with T cell receptors and CD8 molecules. Here we show that paired Ig-like receptor (PIR)-B, an MHC-I receptor expressed on antigen-presenting cells, can regulate CTL triggering by blocking the access of CD8 molecules to MHC-I. PIR-B-deficient DCs evoked CTLs more efficiently, leading to accelerated graft and tumor rejection. PIR-B(+) non-DC transfectant cells served as less efficient stimulators and targets for CTLs than PIR-B(-) cells at the effector phase in vitro. On surface plasmon resonance analysis, PIR-B and CD8alpha alpha were revealed to compete in binding to MHC-I. Our results may provide a novel strategy for regulating CTL-mediated immunity and diseases in a sterical manner.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


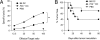
Similar articles
-
Anatomic location and T-cell stimulatory functions of mouse dendritic cell subsets defined by CD4 and CD8 expression.Blood. 2002 Mar 15;99(6):2084-93. doi: 10.1182/blood.v99.6.2084. Blood. 2002. PMID: 11877283
-
Two distinct populations of primary cytotoxic cells infiltrating into allografted tumor rejection sites: infiltration of macrophages cytotoxic against allografted tumor precedes that of multiple sets of cytotoxic T lymphocytes with distinct specificity to alloantigens.Microbiol Immunol. 1997;41(2):149-59. doi: 10.1111/j.1348-0421.1997.tb01180.x. Microbiol Immunol. 1997. PMID: 9087957
-
NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo.J Immunol. 2003 Mar 1;170(5):2540-8. doi: 10.4049/jimmunol.170.5.2540. J Immunol. 2003. PMID: 12594280
-
A novel recognition system for MHC class I molecules constituted by PIR.Adv Immunol. 2005;88:161-92. doi: 10.1016/S0065-2776(05)88005-8. Adv Immunol. 2005. PMID: 16227090 Review.
-
Paired immunoglobulin-like receptors and their MHC class I recognition.Immunology. 2005 Aug;115(4):433-40. doi: 10.1111/j.1365-2567.2005.02177.x. Immunology. 2005. PMID: 16011512 Free PMC article. Review.
Cited by
-
Immunoglobulin-like transcript receptors on human dermal CD14+ dendritic cells act as a CD8-antagonist to control cytotoxic T cell priming.Proc Natl Acad Sci U S A. 2012 Nov 13;109(46):18885-90. doi: 10.1073/pnas.1205785109. Epub 2012 Oct 29. Proc Natl Acad Sci U S A. 2012. PMID: 23112154 Free PMC article. Clinical Trial.
-
Targeting immune checkpoints on myeloid cells: current status and future directions.Cancer Immunol Immunother. 2025 Jan 3;74(2):40. doi: 10.1007/s00262-024-03856-6. Cancer Immunol Immunother. 2025. PMID: 39751898 Free PMC article. Review.
-
LILRB3 (ILT5) is a myeloid cell checkpoint that elicits profound immunomodulation.JCI Insight. 2020 Sep 1;5(18):e141593. doi: 10.1172/jci.insight.141593. JCI Insight. 2020. PMID: 32870822 Free PMC article.
-
Dual functions of angiopoietin-like protein 2 signaling in tumor progression and anti-tumor immunity.Genes Dev. 2019 Dec 1;33(23-24):1641-1656. doi: 10.1101/gad.329417.119. Epub 2019 Nov 14. Genes Dev. 2019. PMID: 31727773 Free PMC article.
-
Structure of MHC class I-like MILL2 reveals heparan-sulfate binding and interdomain flexibility.Nat Commun. 2018 Oct 18;9(1):4330. doi: 10.1038/s41467-018-06797-8. Nat Commun. 2018. PMID: 30337538 Free PMC article.
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. - PubMed
-
- Guermonprez P, et al. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. - PubMed
-
- Cresswell P, et al. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145–157. - PubMed
-
- Gao GF, et al. Crystal structure of the complex between human CD8αα and HLA-A2. Nature. 1997;387:630–634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

