Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma
- PMID: 18787175
- PMCID: PMC2645533
- DOI: 10.1165/rcmb.2008-0276OC
Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma
Abstract
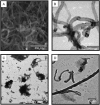
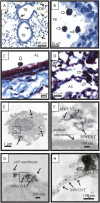
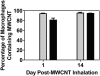
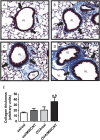
Carbon nanotubes are gaining increasing attention due to possible health risks from occupational or environmental exposures. This study tested the hypothesis that inhaled multiwalled carbon nanotubes (MWCNT) would increase airway fibrosis in mice with allergic asthma. Normal and ovalbumin-sensitized mice were exposed to a MWCNT aerosol (100 mg/m(3)) or saline aerosol for 6 hours. Lung injury, inflammation, and fibrosis were examined by histopathology, clinical chemistry, ELISA, or RT-PCR for cytokines/chemokines, growth factors, and collagen at 1 and 14 days after inhalation. Inhaled MWCNT were distributed throughout the lung and found in macrophages by light microscopy, but were also evident in epithelial cells by electron microscopy. Quantitative morphometry showed significant airway fibrosis at 14 days in mice that received a combination of ovalbumin and MWCNT, but not in mice that received ovalbumin or MWCNT only. Ovalbumin-sensitized mice that did not inhale MWCNT had elevated levels IL-13 and transforming growth factor (TGF)-beta1 in lung lavage fluid, but not platelet-derived growth factor (PDGF)-AA. In contrast, unsensitized mice that inhaled MWCNT had elevated PDGF-AA, but not increased levels of TGF-beta1 and IL-13. This suggested that airway fibrosis resulting from combined ovalbumin sensitization and MWCNT inhalation requires PDGF, a potent fibroblast mitogen, and TGF-beta1, which stimulates collagen production. Combined ovalbumin sensitization and MWCNT inhalation also synergistically increased IL-5 mRNA levels, which could further contribute to airway fibrosis. These data indicate that inhaled MWCNT require pre-existing inflammation to cause airway fibrosis. Our findings suggest that individuals with pre-existing allergic inflammation may be susceptible to airway fibrosis from inhaled MWCNT.
Figures


References
-
- Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, Alexander A. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci 2006;92:5–22. - PubMed
-
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, Stone V, Brown S, MacNee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotech 2008;3:423–428. - PubMed
-
- Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci 2004;77:126–134. - PubMed
-
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol 2005;289:L698–L708. - PubMed
-
- Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci 2004;77:117–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

