Rhinovirus disrupts the barrier function of polarized airway epithelial cells
- PMID: 18787220
- PMCID: PMC2599868
- DOI: 10.1164/rccm.200801-136OC
Rhinovirus disrupts the barrier function of polarized airway epithelial cells
Abstract
Rationale: Secondary bacterial infection following rhinovirus (RV) infection has been recognized in chronic obstructive pulmonary disease.
Objectives: We sought to understand mechanisms by which RV infection facilitates secondary bacterial infection.
Methods: Primary human airway epithelial cells grown at air-liquid interface and human bronchial epithelial (16HBE14o-) cells grown as polarized monolayers were infected apically with RV. Transmigration of bacteria (nontypeable Haemophilus influenzae and others) was assessed by colony counting and transmission electron microscopy. Transepithelial resistance (R(T)) was measured by using a voltmeter. The distribution of zona occludins (ZO)-1 was determined by immunohistochemistry and immunoblotting.
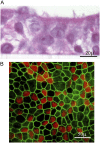
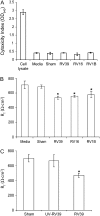
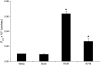
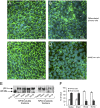
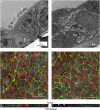
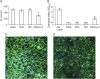
Measurements and main results: Epithelial cells infected with RV showed 2-log more bound bacteria than sham-infected cultures, and bacteria were recovered from the basolateral media of RV- but not sham-infected cells. Infection of polarized airway epithelial cell cultures with RV for 24 hours caused a significant decrease in R(T) without causing cell death or apoptosis. Ultraviolet-treated RV did not decrease R(T), suggesting a requirement for viral replication. Reduced R(T) was associated with increased paracellular permeability, as determined by flux of fluorescein isothiocyanate (FITC)-inulin. Neutralizing antibodies to tumor necrosis factor (TNF)-alpha, IFN-gamma and IL-1beta reversed corresponding cytokine-induced reductions in R(T) but not that induced by RV, indicating that the RV effect is independent of these proinflammatory cytokines. Confocal microscopy and immunoblotting revealed the loss of ZO-1 from tight junction complexes in RV-infected cells. Intranasal inoculation of mice with RV1B also caused the loss of ZO-1 from the bronchial epithelium tight junctions in vivo.
Conclusions: RV facilitates binding, translocation, and persistence of bacteria by disrupting airway epithelial barrier function.
Figures



Similar articles
-
Rhinovirus-induced barrier dysfunction in polarized airway epithelial cells is mediated by NADPH oxidase 1.J Virol. 2011 Jul;85(13):6795-808. doi: 10.1128/JVI.02074-10. Epub 2011 Apr 20. J Virol. 2011. PMID: 21507984 Free PMC article.
-
Rhinovirus infection-induced alteration of tight junction and adherens junction components in human nasal epithelial cells.Laryngoscope. 2010 Feb;120(2):346-52. doi: 10.1002/lary.20764. Laryngoscope. 2010. PMID: 20013846
-
Euphorbium compositum SN improves the innate defenses of the airway mucosal barrier network during rhinovirus infection.Respir Res. 2024 Nov 13;25(1):407. doi: 10.1186/s12931-024-03030-7. Respir Res. 2024. PMID: 39538325 Free PMC article.
-
Rhinoviruses in the pathogenesis of asthma: the bronchial epithelium as a major disease target.J Allergy Clin Immunol. 2006 Sep;118(3):587-90. doi: 10.1016/j.jaci.2006.06.023. J Allergy Clin Immunol. 2006. PMID: 16950275 Review. No abstract available.
-
Are rhinoviruses implicated in the pathogenesis of sinusitis and chronic rhinosinusitis exacerbations? A comprehensive review.Int Forum Allergy Rhinol. 2019 Oct;9(10):1159-1188. doi: 10.1002/alr.22403. Epub 2019 Aug 20. Int Forum Allergy Rhinol. 2019. PMID: 31430424
Cited by
-
Virus-induced modulation of lower airway diseases: pathogenesis and pharmacologic approaches to treatment.Pharmacol Ther. 2015 Apr;148:185-98. doi: 10.1016/j.pharmthera.2014.12.005. Epub 2014 Dec 27. Pharmacol Ther. 2015. PMID: 25550230 Free PMC article. Review.
-
Applications of the phytomedicine Echinacea purpurea (Purple Coneflower) in infectious diseases.J Biomed Biotechnol. 2012;2012:769896. doi: 10.1155/2012/769896. Epub 2011 Oct 26. J Biomed Biotechnol. 2012. PMID: 22131823 Free PMC article. Review.
-
Host-Pathogen Responses to Pandemic Influenza H1N1pdm09 in a Human Respiratory Airway Model.Viruses. 2020 Jun 24;12(6):679. doi: 10.3390/v12060679. Viruses. 2020. PMID: 32599823 Free PMC article.
-
Viral and bacterial infection in acute asthma and chronic obstructive pulmonary disease increases the risk of readmission.Respirology. 2013 Aug;18(6):996-1002. doi: 10.1111/resp.12099. Respirology. 2013. PMID: 23600594 Free PMC article.
-
Interleukin-13 Alters Tight Junction Proteins Expression Thereby Compromising Barrier Function and Dampens Rhinovirus Induced Immune Responses in Nasal Epithelium.Front Cell Dev Biol. 2020 Sep 25;8:572749. doi: 10.3389/fcell.2020.572749. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33102478 Free PMC article.
References
-
- Bonzel L, Tenenbaum T, Schroten H, Schildgen O, Schweitzer-Krantz S, Adams O. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. Pediatr Infect Dis J 2008;27:589–594. - PubMed
-
- Yano H, Okitsu N, Hori T, Watanabe O, Kisu T, Hatagishi E, Suzuki A, Okamoto M, Ohmi A, Suetake M, et al. Detection of respiratory viruses in nasopharyngeal secretions and middle ear fluid from children with acute otitis media. Acta Otolaryngol 2008;June 13:1–6. - PubMed
-
- Greenberg SB, Allen M, Wilson J, Atmar RL. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:167–173. - PubMed
-
- Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries DJ, Johnston SL et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1618–1623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

