A study of the safety and efficacy of calcipotriol and betamethasone dipropionate scalp formulation in the long-term management of scalp psoriasis
- PMID: 18787325
- PMCID: PMC2790732
- DOI: 10.1159/000155642
A study of the safety and efficacy of calcipotriol and betamethasone dipropionate scalp formulation in the long-term management of scalp psoriasis
Abstract
Background: Effective and safe products are needed for long-term management of scalp psoriasis. This study investigated the long-term safety and efficacy of a two-compound formulation (calcipotriol 50 microg/g plus betamethasone dipropionate 0.5 mg/g) for scalp psoriasis.
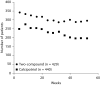
Methods: In this 52-week, international, double-blind study, 869 patients with moderate-to-severe scalp psoriasis were randomized to either a two-compound scalp formulation (n = 429) or calcipotriol (n = 440).
Results: Adverse drug reactions were less frequent in the two-compound group compared with the calcipotriol group (17.2 vs. 29.5%; p < 0.001). Incidences of adverse events possibly associated with long-term corticosteroid use were low in both the two-compound (2.6%) and the calcipotriol (3.0%) groups. Disease was satisfactorily controlled in 92.3% of visits in the two-compound group versus 80.0% in the calcipotriol group (p < 0.001).
Conclusion: The two-compound scalp formulation demonstrated a high level of safety and efficacy in long-term management of scalp psoriasis.
2008 S. Karger AG, Basel.
Figures

References
-
- Farber EM, Nall L. Natural history and treatment of scalp psoriasis. Cutis. 1992;49:396–400. - PubMed
-
- Van de Kerkhof PC, Franssen ME. Psoriasis of the scalp. Diagnosis and management. Am J Clin Dermatol. 2001;2:159–165. - PubMed
-
- Van de Kerkhof PC, de Hoop D, de Korte J, Kuipers MV. Scalp psoriasis, clinical presentations and therapeutic management. Dermatology. 1998;197:326–334. - PubMed
-
- Van de Kerkhof PC, de Hoop D, de Korte J, Cobelens SA, Kuipers MV. Patient compliance and disease management in the treatment of psoriasis in the Netherlands. Dermatology. 2000;200:292–298. - PubMed
-
- Feldman SR, Housman TS. Patients' vehicle preference for corticosteroid treatments of scalp psoriasis. Am J Clin Dermatol. 2003;4:221–224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

