Structural insight into the inhibition of tubulin by vinca domain peptide ligands
- PMID: 18787557
- PMCID: PMC2581847
- DOI: 10.1038/embor.2008.171
Structural insight into the inhibition of tubulin by vinca domain peptide ligands
Abstract
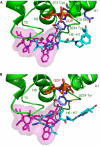
The tubulin vinca domain is the target of widely different microtubule inhibitors that interfere with the binding of vinblastine. Although all these ligands inhibit the hydrolysis of GTP, they affect nucleotide exchange to variable extents. The structures of two vinca domain antimitotic peptides--phomopsin A and soblidotin (a dolastatin 10 analogue)--bound to tubulin in a complex with a stathmin-like domain show that their sites partly overlap with that of vinblastine and extend the definition of the vinca domain. The structural data, together with the biochemical results from the ligands we studied, highlight two main contributors in nucleotide exchange: the flexibility of the tubulin subunits' arrangement at their interfaces and the residues in the carboxy-terminal part of the beta-tubulin H6-H7 loop. The structures also highlight common features of the mechanisms by which vinca domain ligands favour curved tubulin assemblies and destabilize microtubules.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Binding of dolastatin 10 to tubulin at a distinct site for peptide antimitotic agents near the exchangeable nucleotide and vinca alkaloid sites.J Biol Chem. 1990 Oct 5;265(28):17141-9. J Biol Chem. 1990. PMID: 2211617
-
Structural plasticity of tubulin assembly probed by vinca-domain ligands.Acta Crystallogr D Biol Crystallogr. 2012 Aug;68(Pt 8):927-34. doi: 10.1107/S0907444912017143. Epub 2012 Jul 7. Acta Crystallogr D Biol Crystallogr. 2012. PMID: 22868758
-
Dolastatin 10, a powerful cytostatic peptide derived from a marine animal. Inhibition of tubulin polymerization mediated through the vinca alkaloid binding domain.Biochem Pharmacol. 1990 Jun 15;39(12):1941-9. doi: 10.1016/0006-2952(90)90613-p. Biochem Pharmacol. 1990. PMID: 2353935
-
The binding of vinca domain agents to tubulin: structural and biochemical studies.Methods Cell Biol. 2010;95:373-90. doi: 10.1016/S0091-679X(10)95020-6. Methods Cell Biol. 2010. PMID: 20466145 Review.
-
Effects of antimitotic agents on tubulin-nucleotide interactions.Pharmacol Ther. 1991 Nov;52(2):127-47. doi: 10.1016/0163-7258(91)90004-6. Pharmacol Ther. 1991. PMID: 1818332 Review.
Cited by
-
Microtubule targeting agents: from biophysics to proteomics.Cell Mol Life Sci. 2010 Apr;67(7):1089-104. doi: 10.1007/s00018-009-0245-6. Epub 2010 Jan 28. Cell Mol Life Sci. 2010. PMID: 20107862 Free PMC article. Review.
-
(-)-Rhazinilam and the diphenylpyridazinone NSC 613241: Two compounds inducing the formation of morphologically similar tubulin spirals but binding apparently to two distinct sites on tubulin.Arch Biochem Biophys. 2016 Aug 15;604:63-73. doi: 10.1016/j.abb.2016.06.008. Epub 2016 Jun 13. Arch Biochem Biophys. 2016. PMID: 27311615 Free PMC article.
-
Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules.Mol Cancer Ther. 2010 Oct;9(10):2689-99. doi: 10.1158/1535-7163.MCT-10-0644. Mol Cancer Ther. 2010. PMID: 20937594 Free PMC article.
-
Variations in the colchicine-binding domain provide insight into the structural switch of tubulin.Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):13775-9. doi: 10.1073/pnas.0904223106. Epub 2009 Aug 5. Proc Natl Acad Sci U S A. 2009. PMID: 19666559 Free PMC article.
-
Biosynthetic investigation of phomopsins reveals a widespread pathway for ribosomal natural products in Ascomycetes.Proc Natl Acad Sci U S A. 2016 Mar 29;113(13):3521-6. doi: 10.1073/pnas.1522907113. Epub 2016 Mar 15. Proc Natl Acad Sci U S A. 2016. PMID: 26979951 Free PMC article.
References
-
- Amayed P, Carlier MF, Pantaloni D (2000) Stathmin slows down guanosine diphosphate dissociation from tubulin in a phosphorylation-controlled fashion. Biochemistry 39: 12295–12302 - PubMed
-
- Bai RL, Pettit GR, Hamel E (1990a) Binding of dolastatin 10 to tubulin at a distinct site for peptide antimitotic agents near the exchangeable nucleotide and vinca alkaloid sites. J Biol Chem 265: 17141–17149 - PubMed
-
- Bai RL, Pettit GR, Hamel E (1990b) Structure–activity studies with chiral isomers and with segments of the antimitotic marine peptide dolastatin 10. Biochem Pharmacol 40: 1859–1864 - PubMed
-
- Bai R, Roach MC, Jayaram SK, Barkoczy J, Pettit GR, Luduena RF, Hamel E (1993) Differential effects of active isomers, segments, and analogs of dolastatin 10 on ligand interactions with tubulin. Correlation with cytotoxicity. Biochem Pharmacol 45: 1503–1515 - PubMed
-
- Bai R, Durso NA, Sackett DL, Hamel E (1999) Interactions of the sponge-derived antimitotic tripeptide hemiasterlin with tubulin: comparison with dolastatin 10 and cryptophycin 1. Biochemistry 38: 14302–14310 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

