The zinc-binding protein Hot13 promotes oxidation of the mitochondrial import receptor Mia40
- PMID: 18787558
- PMCID: PMC2581857
- DOI: 10.1038/embor.2008.173
The zinc-binding protein Hot13 promotes oxidation of the mitochondrial import receptor Mia40
Abstract
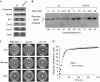
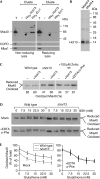
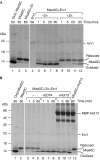
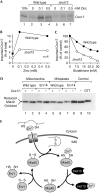
A disulphide relay system mediates the import of cysteine-containing proteins into the intermembrane space of mitochondria. This system consists of two essential proteins, Mia40 and Erv1, which bind to newly imported proteins by disulphide transfer. A third component, Hot13, was proposed to be important in the biogenesis of cysteine-rich proteins of the intermembrane space, but the molecular function of Hot13 remained unclear. Here, we show that Hot13, a conserved zinc-binding protein, interacts functionally and physically with the import receptor Mia40. It improves the Erv1-dependent oxidation of Mia40 both in vivo and in vitro. As a consequence, in mutants lacking Hot13, the import of substrates of Mia40 is impaired, particularly in the presence of zinc ions. In mitochondria as well as in vitro, Hot13 can be functionally replaced by zinc-binding chelators. We propose that Hot13 maintains Mia40 in a zinc-free state, thereby facilitating its efficient oxidation by Erv1.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
The N-terminal shuttle domain of Erv1 determines the affinity for Mia40 and mediates electron transfer to the catalytic Erv1 core in yeast mitochondria.Antioxid Redox Signal. 2010 Nov 1;13(9):1327-39. doi: 10.1089/ars.2010.3200. Antioxid Redox Signal. 2010. PMID: 20367271
-
Structural and functional roles of the conserved cysteine residues of the redox-regulated import receptor Mia40 in the intermembrane space of mitochondria.J Biol Chem. 2009 Jan 16;284(3):1353-63. doi: 10.1074/jbc.M805035200. Epub 2008 Nov 14. J Biol Chem. 2009. PMID: 19011240
-
Precursor oxidation by Mia40 and Erv1 promotes vectorial transport of proteins into the mitochondrial intermembrane space.Mol Biol Cell. 2008 Jan;19(1):226-36. doi: 10.1091/mbc.e07-08-0814. Epub 2007 Oct 31. Mol Biol Cell. 2008. PMID: 17978092 Free PMC article.
-
A disulfide relay system in mitochondria.Cell. 2005 Jul 1;121(7):965-7. doi: 10.1016/j.cell.2005.06.019. Cell. 2005. PMID: 15989945 Review.
-
Structural basis for the disulfide relay system in the mitochondrial intermembrane space.Antioxid Redox Signal. 2010 Nov 1;13(9):1359-73. doi: 10.1089/ars.2010.3099. Antioxid Redox Signal. 2010. PMID: 20136511 Review.
Cited by
-
The Mia40/CHCHD4 Oxidative Folding System: Redox Regulation and Signaling in the Mitochondrial Intermembrane Space.Antioxidants (Basel). 2021 Apr 12;10(4):592. doi: 10.3390/antiox10040592. Antioxidants (Basel). 2021. PMID: 33921425 Free PMC article. Review.
-
The power of yeast to model diseases of the powerhouse of the cell.Front Biosci (Landmark Ed). 2013 Jan 1;18(1):241-78. doi: 10.2741/4098. Front Biosci (Landmark Ed). 2013. PMID: 23276920 Free PMC article. Review.
-
Cytosolic redox components regulate protein homeostasis via additional localisation in the mitochondrial intermembrane space.FEBS Lett. 2017 Sep;591(17):2661-2670. doi: 10.1002/1873-3468.12766. Epub 2017 Aug 6. FEBS Lett. 2017. PMID: 28746987 Free PMC article. Review.
-
Kinetics and mechanisms of thiol-disulfide exchange covering direct substitution and thiol oxidation-mediated pathways.Antioxid Redox Signal. 2013 May 1;18(13):1623-41. doi: 10.1089/ars.2012.4973. Epub 2013 Jan 9. Antioxid Redox Signal. 2013. PMID: 23075118 Free PMC article. Review.
-
The Journey of Mitochondrial Protein Import and the Roadmap to Follow.Int J Mol Sci. 2023 Jan 27;24(3):2479. doi: 10.3390/ijms24032479. Int J Mol Sci. 2023. PMID: 36768800 Free PMC article. Review.
References
-
- Allen S, Balabanidou V, Sideris DP, Lisowsky T, Tokatlidis K (2005) Erv1 mediates the Mia40-dependent protein import pathway and provides a functional link to the respiratory chain by shuttling electrons to cytochrome c. J Mol Biol 353: 937–944 - PubMed
-
- Altmann K, Dürr M, Westermann B (2007) Saccharomyces cerevisiae as a model organism to study mitochondrial biology. In Mitochondria. Practical Protocols, Leister D, Herrmann JM (eds) Vol 372, pp 81–90. Totowa, New Jersey, USA: Humana - PubMed
-
- Curran SP, Leuenberger D, Leverich EP, Hwang DK, Beverly KN, Koehler CM (2004) The role of Hot13p and redox chemistry in the mitochondrial TIM22 import pathway. J Biol Chem 279: 43744–43751 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

