Safety and efficacy of rivastigmine in patients with Alzheimer's disease not responding adequately to donepezil: an open-label study
- PMID: 18787673
- PMCID: PMC2528233
- DOI: 10.4088/pcc.v10n0404
Safety and efficacy of rivastigmine in patients with Alzheimer's disease not responding adequately to donepezil: an open-label study
Abstract
Objective: Switching patients with Alzheimer's disease from one cholinesterase inhibitor to another represents a viable option for patients not responding to current therapy. The objective of this large U.S.-based study was to evaluate the safety and efficacy of a treatment switch to rivastigmine in patients not responding adequately to or declining on treatment with donepezil.
Method: In this 26-week, prospective, open-label, single-arm, multicenter study conducted from April 24, 2003, to June 25, 2004, patients with mild-to-moderate Alzheimer's disease (DSM-IV-TR criteria) who were not responding to donepezil were treated with rivastigmine 3-12 mg/day. Safety and tolerability were measured by the occurrence of adverse events and patient disposition. Treatment effects on global functioning were assessed using the Clinical Global Impression of Change (CGIC) scale.
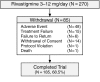
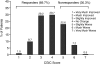
Results: Two hundred seventy patients with a mean age of 78.5 (SD = 7.56) years and a mean duration of dementia of 3.5 (SD = 2.06) years were included in the study. Sixty-nine percent of patients completed the study with 17.8% discontinuing due to adverse events. Eighty-three percent of patients reported at least 1 adverse event, with the most frequently occurring adverse events affecting the gastrointestinal system (54%). The majority of patients were reported to have either improvement or no decline on the CGIC. A limitation of the study is that the interpretation of the results is based on an overall completion rate of 69%.
Conclusion: Immediately switching patients from donepezil to rivastigmine without a washout period was safe and well tolerated in the current study. Additionally, these results suggest that patients not responding adequately to or declining while taking donepezil may improve or stabilize after switching to rivastigmine.
Figures

References
-
- Doody RS, Stevens JC, and Beck C. et al. Practice parameter: management of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001 56:1154–1166. - PubMed
-
- Ellis JM.. Cholinesterase inhibitors in the treatment of dementia. J Am Osteopath Assoc. 2005;105:145–158. - PubMed
-
- Gauthier S, Emre M, and Farlow MR. et al. Strategies for continued successful treatment of Alzheimer's disease: switching cholinesterase inhibitors. Curr Med Res Opin. 2003 19:707–714. - PubMed
-
- Auriacombe S, Pere JJ, and Loria-Kanza Y. et al. Efficacy and safety of rivastigmine in patients with Alzheimer's disease who failed to benefit from treatment with donepezil. Curr Med Res Opin. 2002 18:129–138. - PubMed
-
- Bullock R, Connolly C.. Switching cholinesterase inhibitor therapy in Alzheimer's disease: donepezil to rivastigmine, is it worth it? Int J Geriatr Psychiatry. 2002;17:288–289. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
