Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses
- PMID: 18787692
- PMCID: PMC2522277
- DOI: 10.1371/journal.ppat.1000151
Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses
Abstract
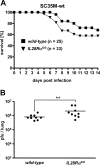
Virus-infected cells secrete a broad range of interferon (IFN) subtypes which in turn trigger the synthesis of antiviral factors that confer host resistance. IFN-alpha, IFN-beta and other type I IFNs signal through a common universally expressed cell surface receptor, whereas IFN-lambda uses a distinct receptor complex for signaling that is not present on all cell types. Since type I IFN receptor-deficient mice (IFNAR1(0/0)) exhibit greatly increased susceptibility to various viral diseases, it remained unclear to which degree IFN-lambda might contribute to innate immunity. To address this issue we performed influenza A virus infections of mice which carry functional alleles of the influenza virus resistance gene Mx1 and which, therefore, develop a more complete innate immune response to influenza viruses than standard laboratory mice. We demonstrate that intranasal administration of IFN-lambda readily induced the antiviral factor Mx1 in mouse lungs and efficiently protected IFNAR1(0/0) mice from lethal influenza virus infection. By contrast, intraperitoneal application of IFN-lambda failed to induce Mx1 in the liver of IFNAR1(0/0) mice and did not protect against hepatotropic virus infections. Mice lacking functional IFN-lambda receptors were only slightly more susceptible to influenza virus than wild-type mice. However, mice lacking functional receptors for both IFN-alpha/beta and IFN-lambda were hypersensitive and even failed to restrict usually non-pathogenic influenza virus mutants lacking the IFN-antagonistic factor NS1. Interestingly, the double-knockout mice were not more susceptible against hepatotropic viruses than IFNAR1(0/0) mice. From these results we conclude that IFN-lambda contributes to inborn resistance against viral pathogens infecting the lung but not the liver.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Yoneyama M, Fujita T. Function of RIG-I-like receptors in antiviral innate immunity. J Biol Chem. 2007;282:15315–15318. - PubMed
-
- Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. - PubMed
-
- Uematsu S, Akira S. Toll-like receptors and Type I interferons. J Biol Chem. 2007;282:15319–15323. - PubMed
-
- Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, et al. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34:796–805. - PubMed
-
- Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

