The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure
- PMID: 18787701
- PMCID: PMC2527135
- DOI: 10.1371/journal.pgen.1000190
The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure
Abstract
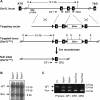
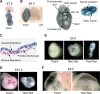
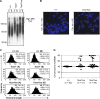
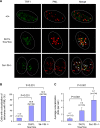
Dot1 is an evolutionarily conserved histone methyltransferase specific for lysine 79 of histone H3 (H3K79). In Saccharomyces cerevisiae, Dot1-mediated H3K79 methylation is associated with telomere silencing, meiotic checkpoint control, and DNA damage response. The biological function of H3K79 methylation in mammals, however, remains poorly understood. Using gene targeting, we generated mice deficient for Dot1L, the murine Dot1 homologue. Dot1L-deficient embryos show multiple developmental abnormalities, including growth impairment, angiogenesis defects in the yolk sac, and cardiac dilation, and die between 9.5 and 10.5 days post coitum. To gain insights into the cellular function of Dot1L, we derived embryonic stem (ES) cells from Dot1L mutant blastocysts. Dot1L-deficient ES cells show global loss of H3K79 methylation as well as reduced levels of heterochromatic marks (H3K9 di-methylation and H4K20 tri-methylation) at centromeres and telomeres. These changes are accompanied by aneuploidy, telomere elongation, and proliferation defects. Taken together, these results indicate that Dot1L and H3K79 methylation play important roles in heterochromatin formation and in embryonic development.
Conflict of interest statement
All authors except AB, SG, and YZ are employees of Novartis Institutes for Biomedical Research.
Figures

Similar articles
-
The histone methyltransferase Dot1/DOT1L as a critical regulator of the cell cycle.Cell Cycle. 2014;13(5):726-38. doi: 10.4161/cc.28104. Epub 2014 Feb 6. Cell Cycle. 2014. PMID: 24526115 Free PMC article. Review.
-
Deficiency of H3K79 histone methyltransferase Dot1-like protein (DOT1L) inhibits cell proliferation.J Biol Chem. 2012 Feb 17;287(8):5588-99. doi: 10.1074/jbc.M111.328138. Epub 2011 Dec 21. J Biol Chem. 2012. PMID: 22190683 Free PMC article.
-
Dot1L mediated histone H3 lysine79 methylation is essential to meiosis progression in mouse oocytes.Neuro Endocrinol Lett. 2014;35(6):523-30. Neuro Endocrinol Lett. 2014. PMID: 25433842
-
DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells.Mol Cell Biol. 2008 Apr;28(8):2825-39. doi: 10.1128/MCB.02076-07. Epub 2008 Feb 19. Mol Cell Biol. 2008. PMID: 18285465 Free PMC article.
-
The diverse functions of Dot1 and H3K79 methylation.Genes Dev. 2011 Jul 1;25(13):1345-58. doi: 10.1101/gad.2057811. Genes Dev. 2011. PMID: 21724828 Free PMC article. Review.
Cited by
-
DOT1L is a barrier to histone acetylation during reprogramming to pluripotency.Sci Adv. 2023 Nov 15;9(46):eadf3980. doi: 10.1126/sciadv.adf3980. Epub 2023 Nov 17. Sci Adv. 2023. PMID: 37976354 Free PMC article.
-
Targeting histone methylation for cancer therapy: enzymes, inhibitors, biological activity and perspectives.J Hematol Oncol. 2016 Jun 17;9(1):49. doi: 10.1186/s13045-016-0279-9. J Hematol Oncol. 2016. PMID: 27316347 Free PMC article. Review.
-
DOT-1.1-dependent H3K79 methylation promotes normal meiotic progression and meiotic checkpoint function in C. elegans.PLoS Genet. 2020 Oct 26;16(10):e1009171. doi: 10.1371/journal.pgen.1009171. eCollection 2020 Oct. PLoS Genet. 2020. PMID: 33104701 Free PMC article.
-
Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis.Proc Natl Acad Sci U S A. 2012 May 22;109(21):8218-23. doi: 10.1073/pnas.1119899109. Epub 2012 May 7. Proc Natl Acad Sci U S A. 2012. PMID: 22566624 Free PMC article.
-
Local enrichment of HP1alpha at telomeres alters their structure and regulation of telomere protection.Nat Commun. 2018 Sep 4;9(1):3583. doi: 10.1038/s41467-018-05840-y. Nat Commun. 2018. PMID: 30181605 Free PMC article.
References
-
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. - PubMed
-
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. - PubMed
-
- Feng Q, Wang H, Ng HH, Erdjument-Bromage H, Tempst P, et al. Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr Biol. 2002;12:1052–1058. - PubMed
-
- Janzen CJ, Hake SB, Lowell JE, Cross GA. Selective di- or trimethylation of histone H3 lysine 76 by two DOT1 homologs is important for cell cycle regulation in Trypanosoma brucei. Mol Cell. 2006;23:497–507. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

