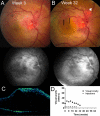
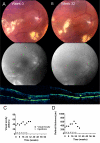
Intravitreal ranibizumab therapy for retinal capillary hemangioblastoma related to von Hippel-Lindau disease
- PMID: 18789534
- PMCID: PMC3034164
- DOI: 10.1016/j.ophtha.2008.04.033
Intravitreal ranibizumab therapy for retinal capillary hemangioblastoma related to von Hippel-Lindau disease
Abstract
Purpose: To evaluate the effect of intravitreal ranibizumab on retinal capillary hemangioblastomas (RCHs) associated with von Hippel-Lindau (VHL) disease that are not amenable or responsive to standard therapy.
Design: Prospective, noncomparative, interventional case series.
Participants: Five patients with VHL-associated RCH with exudative changes and visual loss.
Methods: Monthly intravitreal injections of ranibizumab (0.5 mg) were given over a course of 6 months for a total of 7 injections, with additional injections considered until week 52. The final study visit was designated as 8 weeks after the final study injection.
Main outcome measures: The primary outcome was the change in best-corrected visual acuity (BCVA) of >/=15 letters at the final visit compared with baseline. Secondary outcomes included change in lesion size, exudation as assessed clinically and by fluorescein angiography, change in retinal thickness as evaluated by optical coherence tomography, and adverse event assessments.
Results: Patients received an average of 10.0+/-3.1 injections over an average period of 47+/-14 weeks, including follow-up. Mean change in BCVA was a decrease of 9+/-20 letters, with 1 patient gaining >/=15 letters, and 2 patients losing >/=15 letters. Changes in both lesion size and exudation were variable.
Conclusions: Intravitreal ranibizumab, delivered as monotherapy every 4 weeks, had minimal beneficial effects on most VHL-related RCHs. Possible treatment efficacy was demonstrated in the patient with the smallest lesion with less exudation. Future prospective studies are needed to determine the potential role of an antiangiogenic agent, possibly in combination with other therapies for the treatment of such advanced ocular tumors associated with VHL.
Figures
Similar articles
-
Intravitreal anti-vascular endothelial growth factor therapy with pegaptanib for advanced von Hippel-Lindau disease of the retina.Retina. 2007 Feb;27(2):150-8. doi: 10.1097/IAE.0b013e318030a290. Retina. 2007. PMID: 17290195 Clinical Trial.
-
[Intravitreal anti-VEGF therapy for capillary hemangioblastomas in von Hippel-Lindau disease].Klin Monbl Augenheilkd. 2008 Apr;225(4):292-4. doi: 10.1055/s-2008-1027175. Klin Monbl Augenheilkd. 2008. PMID: 18401796 German.
-
Intravitreous treatment of severe ocular von Hippel-Lindau disease using a combination of the VEGF inhibitor, ranibizumab and PDGF inhibitor, E10030: Results from a phase 1/2 clinical trial.Clin Exp Ophthalmol. 2021 Dec;49(9):1048-1059. doi: 10.1111/ceo.14001. Epub 2021 Oct 26. Clin Exp Ophthalmol. 2021. PMID: 34549489 Free PMC article. Clinical Trial.
-
Intravitreal bevacizumab for retinal capillary hemangioblastoma: A case series and literature review.Can J Ophthalmol. 2014 Oct;49(5):450-7. doi: 10.1016/j.jcjo.2014.07.007. Can J Ophthalmol. 2014. PMID: 25284102 Review.
-
Anti-vascular endothelial growth factor for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2019 Mar 4;3(3):CD005139. doi: 10.1002/14651858.CD005139.pub4. Cochrane Database Syst Rev. 2019. PMID: 30834517 Free PMC article.
Cited by
-
Juxtapapillary retinal capillary hemangioma: new therapeutic strategies.Med Hypothesis Discov Innov Ophthalmol. 2014 Fall;3(3):71-5. Med Hypothesis Discov Innov Ophthalmol. 2014. PMID: 25741522 Free PMC article. Review.
-
MANAGEMENT OF RETINAL HEMANGIOBLASTOMA IN VON HIPPEL-LINDAU DISEASE.Retina. 2019 Dec;39(12):2254-2263. doi: 10.1097/IAE.0000000000002572. Retina. 2019. PMID: 31259811 Free PMC article. Review.
-
Genetics, Pathophysiology, and Current Challenges in Von Hippel-Lindau Disease Therapeutics.Diagnostics (Basel). 2024 Aug 29;14(17):1909. doi: 10.3390/diagnostics14171909. Diagnostics (Basel). 2024. PMID: 39272694 Free PMC article. Review.
-
Resolution of epiretinal membrane after anti-VEGF and photodynamic therapy of retinal hemangioblastoma.Am J Ophthalmol Case Rep. 2024 Jan 10;33:101994. doi: 10.1016/j.ajoc.2024.101994. eCollection 2024 Mar. Am J Ophthalmol Case Rep. 2024. PMID: 38303898 Free PMC article.
-
Von Hippel-Lindau Disease and the Eye.J Ophthalmic Vis Res. 2020 Feb 2;15(1):78-94. doi: 10.18502/jovr.v15i1.5950. eCollection 2020 Jan-Mar. J Ophthalmic Vis Res. 2020. PMID: 32095212 Free PMC article. Review.
References
-
- Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–20. - PubMed
-
- Singh AD, Shields CL, Shields JA. von Hippel-Lindau disease. Surv Ophthalmol. 2001;46:117–42. - PubMed
-
- McCabe CM, Flynn HW, Jr., Shields CL, et al. Juxtapapillary capillary hemangiomas. Clinical features and visual acuity outcomes. Ophthalmology. 2000;107:2240–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical