Screening helix-threading peptides for RNA binding using a thiazole orange displacement assay
- PMID: 18789700
- PMCID: PMC2585546
- DOI: 10.1016/j.bmc.2008.08.066
Screening helix-threading peptides for RNA binding using a thiazole orange displacement assay
Abstract
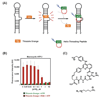
The fluorescent intercalator displacement assay using thiazole orange has been adapted to the study of RNA-binding helix-threading peptides (HTPs). This assay is highly sensitive with HTP-binding RNAs and provides binding affinity data in good agreement with quantitative ribonuclease footprinting without the need for radiolabeling or gel electrophoresis. The FID assay was used to define structure activity relationships for a small library of helix-threading peptides. Results of these studies indicate their RNA binding is dependent on peptide sequence, alpha-amino acid stereochemistry, and cyclization (vs linear peptides), but independent of macrocyclic ring size for the penta-, tetra- and tri-peptides analyzed.
Figures


Similar articles
-
Thiazole orange as the fluorescent intercalator in a high resolution fid assay for determining DNA binding affinity and sequence selectivity of small molecules.Bioorg Med Chem. 2001 Sep;9(9):2511-8. doi: 10.1016/s0968-0896(01)00243-7. Bioorg Med Chem. 2001. PMID: 11553493
-
A modified fluorescent intercalator displacement assay for RNA ligand discovery.Anal Biochem. 2011 Jan 15;408(2):269-76. doi: 10.1016/j.ab.2010.09.020. Epub 2010 Sep 21. Anal Biochem. 2011. PMID: 20863807 Free PMC article.
-
Forced intercalation-induced light-up peptides as fluorogenic indicators for the HIV-1 TAR RNA-ligand assay.Analyst. 2024 Aug 5;149(16):4179-4186. doi: 10.1039/d4an00530a. Analyst. 2024. PMID: 38860915
-
Lysine linkage in abasic site-binding ligand-thiazole orange conjugates for improved binding affinity to orphan nucleobases in DNA/RNA hybrids.Chem Commun (Camb). 2016 Dec 13;52(100):14446-14449. doi: 10.1039/c6cc07236d. Chem Commun (Camb). 2016. PMID: 27901527
-
RNA binding and thiolytic stability of a quinoline-containing helix-threading peptide.Org Biomol Chem. 2006 Feb 21;4(4):639-45. doi: 10.1039/b513591e. Epub 2006 Jan 24. Org Biomol Chem. 2006. PMID: 16467938
Cited by
-
Identifying the preferred RNA motifs and chemotypes that interact by probing millions of combinations.Nat Commun. 2012;3:1125. doi: 10.1038/ncomms2119. Nat Commun. 2012. PMID: 23047683 Free PMC article.
-
Selective Small Molecule Recognition of RNA Base Pairs.ACS Comb Sci. 2018 Aug 13;20(8):482-491. doi: 10.1021/acscombsci.8b00049. Epub 2018 Jul 31. ACS Comb Sci. 2018. PMID: 29966095 Free PMC article.
-
Multivalent binding oligomers inhibit HIV Tat-TAR interaction critical for viral replication.Bioorg Med Chem Lett. 2009 Dec 15;19(24):6893-7. doi: 10.1016/j.bmcl.2009.10.078. Epub 2009 Oct 23. Bioorg Med Chem Lett. 2009. PMID: 19896372 Free PMC article.
-
Small molecule targeting r(UGGAA)n disrupts RNA foci and alleviates disease phenotype in Drosophila model.Nat Commun. 2021 Jan 11;12(1):236. doi: 10.1038/s41467-020-20487-4. Nat Commun. 2021. PMID: 33431896 Free PMC article.
-
Large-scale analysis of small molecule-RNA interactions using multiplexed RNA structure libraries.Commun Chem. 2024 May 1;7(1):98. doi: 10.1038/s42004-024-01181-8. Commun Chem. 2024. PMID: 38693284 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

