Modulation of adaptive immunity by different adjuvant-antigen combinations in mice lacking Nod2
- PMID: 18789992
- PMCID: PMC2586078
- DOI: 10.1016/j.vaccine.2008.08.038
Modulation of adaptive immunity by different adjuvant-antigen combinations in mice lacking Nod2
Abstract
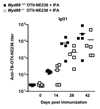
The mechanisms underlying adjuvant effects are under renewed scrutiny because of the enormous implications for vaccine development. Additionally, new low-toxicity adjuvants are sought to enhance vaccine formulations. Muramyl dipeptide (MDP) is a component of the peptidoglycan polymer and was shown to be an active but low-toxicity component of complete Freund's adjuvant, a powerful adjuvant composed of mycobacteria lysates in an oil emulsion. MDP activates cells primarily via the cytosolic NLR family member Nod2 and is therefore linked to the ability of adjuvants to enhance antibody production. Accordingly, we tested the adjuvant properties of the MDP-Nod2 pathway. We found that MDP, compared to the TLR agonist lipopolysaccharide, has minimal adjuvant properties for antibody production under a variety of immunization conditions. We also observed that the oil emulsion incomplete Freund's adjuvant (IFA) supplanted the requirements for the TLR pathway independent of the antigen. Surprisingly, we observed that Nod2 was required for an optimal IgG1 and IgG2c response in the absence of exogenous TLR or NLR agonists. Collectively, our results argue that oil emulsions deserve greater attention for their immunostimulatory properties.
Figures
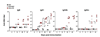

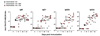
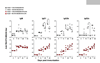
Similar articles
-
Influence of a synthetic adjuvant (MDP) on qualitative and quantitative changes of serum globulins.Immunology. 1978 Dec;35(6):963-70. Immunology. 1978. PMID: 738768 Free PMC article.
-
NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2.J Biol Chem. 2007 Dec 14;282(50):36223-9. doi: 10.1074/jbc.M703079200. Epub 2007 Oct 18. J Biol Chem. 2007. PMID: 17947236
-
Co-adjuvanting Nod2-stimulating bacteria with a TLR7 agonist elicits potent protective immunity against respiratory virus infection.Int J Antimicrob Agents. 2024 Dec;64(6):107369. doi: 10.1016/j.ijantimicag.2024.107369. Epub 2024 Oct 29. Int J Antimicrob Agents. 2024. PMID: 39477030
-
Freund's adjuvant, NOD2 and mycobacteria.Curr Opin Microbiol. 2015 Feb;23:126-32. doi: 10.1016/j.mib.2014.11.015. Epub 2014 Dec 5. Curr Opin Microbiol. 2015. PMID: 25483349 Review.
-
[Adjuvants: update on concepts].Rev Argent Microbiol. 1990 Jul-Sep;22(3):159-66. Rev Argent Microbiol. 1990. PMID: 2102016 Review. Spanish. No abstract available.
Cited by
-
Peptide emulsions in incomplete Freund's adjuvant create effective nurseries promoting egress of systemic CD4+ and CD8+ T cells for immunotherapy of cancer.J Immunother Cancer. 2022 Sep;10(9):e004709. doi: 10.1136/jitc-2022-004709. J Immunother Cancer. 2022. PMID: 36939214 Free PMC article. Review.
-
Beyond the inflammasome: regulatory NOD-like receptor modulation of the host immune response following virus exposure.J Gen Virol. 2016 Apr;97(4):825-838. doi: 10.1099/jgv.0.000401. Epub 2016 Jan 13. J Gen Virol. 2016. PMID: 26763980 Free PMC article. Review.
-
The ever-expanding function of NOD2: autophagy, viral recognition, and T cell activation.Trends Immunol. 2011 Feb;32(2):73-9. doi: 10.1016/j.it.2010.12.007. Epub 2011 Jan 19. Trends Immunol. 2011. PMID: 21251876 Free PMC article.
-
Evaluation of a new oil adjuvant for use in peptide-based cancer vaccination.Cancer Sci. 2010 Oct;101(10):2110-4. doi: 10.1111/j.1349-7006.2010.01653.x. Epub 2010 Jul 30. Cancer Sci. 2010. PMID: 20678155 Free PMC article. Clinical Trial.
-
Macroglobulinemia and Autoinflammatory Disease.Rheumatol Immunol Res. 2021 Dec 31;2(4):227-232. doi: 10.2478/rir-2021-0031. eCollection 2021 Dec. Rheumatol Immunol Res. 2021. PMID: 36467983 Free PMC article. Review.
References
-
- McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27(5):687–690. - PubMed
-
- Dupuis M, McDonald DM, Ott G. Distribution of adjuvant MF59 and antigen gD2 after intramuscular injection in mice. Vaccine. 1999;18(5–6):434–439. - PubMed
-
- O'Hagan DT, Wack A, Podda A. MF59 is a safe and potent vaccine adjuvant for flu vaccines in humans: what did we learn during its development? Clin Pharmacol Ther. 2007;82(6):740–744. - PubMed
-
- Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178(8):5271–5276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

