Addictive drugs modulate GIRK-channel signaling by regulating RGS proteins
- PMID: 18790542
- PMCID: PMC2818288
- DOI: 10.1016/j.tips.2008.07.011
Addictive drugs modulate GIRK-channel signaling by regulating RGS proteins
Abstract
Regulator of G-protein signaling (RGS) proteins are strong modulators of G-protein-mediated pathways in the nervous system. One function of RGS proteins is to accelerate the activation-deactivation kinetics of G-protein-coupled inwardly rectifying potassium (GIRK) channels. The opening of GIRK channels reduces the firing rates of neurons. Recent studies indicate that RGS proteins also modulate the coupling efficiency between gamma-aminobutyric acid type B (GABA(B)) receptors and GIRK channels in dopamine neurons of the ventral tegmental area (VTA), the initial target for addictive drugs in the brain reward pathway. Chronic drug exposure can dynamically regulate the expression levels of RGS. Functional and behavioral studies now reveal that levels of RGS2 protein, through selective association with GIRK3, critically determine whether GABA(B) agonists are excitatory or inhibitory in the VTA. The regulation of RGS protein in the reward pathway might underlie adaptation to different types of addictive drugs.
Figures

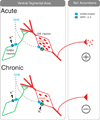
References
-
- Cruz HG, et al. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat. Neurosci. 2004;7:153–159. - PubMed
-
- Labouebe G, et al. RGS2 modulates coupling between GABA(B) receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat. Neurosci. 2007 - PubMed
-
- Stanfield PR, et al. Constitutively active and G-protein coupled inward rectifier K+ channels: Kir2.0 and Kir3.0. Rev. Physiol. Biochem. Pharmacol. 2002;145:47–179. - PubMed
-
- Peleg S, et al. G(alpha)(i) controls the gating of the G protein-activated K(+) channel, GIRK. Neuron. 2002;33:87–99. - PubMed

