Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism
- PMID: 18790744
- PMCID: PMC2596943
- DOI: 10.1158/1535-7163.MCT-07-2400
Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism
Abstract
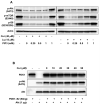
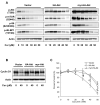
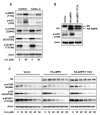
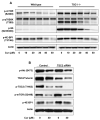
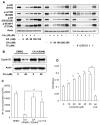
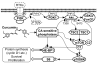
Akt/mammalian target of rapamycin (mTOR) signaling plays an important role in tumorigenesis and is dysregulated in many tumors, especially metastatic prostate cancers. Curcumin has been shown to effectively prevent or inhibit prostate cancer in vivo and inhibit Akt/mTOR signaling in vitro, but the mechanism(s) remains unclear. Here, we show that curcumin concentration- and time-dependently inhibited the phosphorylation of Akt, mTOR, and their downstream substrates in human prostate cancer PC-3 cells, and this inhibitory effect acts downstream of phosphatidylinositol 3-kinase and phosphatidylinositol-dependent kinase 1. Overexpression of constitutively activated Akt or disruption of TSC1-TSC2 complex by small interfering RNA or gene knockout only partially restored curcumin-mediated inhibition of mTOR and downstream signaling, indicating that they are not the primary effectors of curcumin-mediated inhibition of Akt/mTOR signaling. Curcumin also activated 5'-AMP-activated protein kinase and mitogen-activated protein kinases; however, inhibition of these kinases failed to rescue the inhibition by curcumin. Finally, it was shown that the inhibition of Akt/mTOR signaling by curcumin is resulted from calyculin A-sensitive protein phosphatase-dependent dephosphorylation. Our study reveals the profound effects of curcumin on the Akt/mTOR signaling network in PC-3 cells and provides new mechanisms for the anticancer effects of curcumin.
Figures



Similar articles
-
Loss of tuberous sclerosis complex-2 function and activation of mammalian target of rapamycin signaling in endometrial carcinoma.Clin Cancer Res. 2008 May 1;14(9):2543-50. doi: 10.1158/1078-0432.CCR-07-0321. Clin Cancer Res. 2008. PMID: 18451215
-
Activation of mammalian target of rapamycin signaling pathway contributes to tumor cell survival in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma.Cancer Res. 2006 Jul 1;66(13):6589-97. doi: 10.1158/0008-5472.CAN-05-3018. Cancer Res. 2006. PMID: 16818631 Free PMC article.
-
Fibronectin stimulates non-small cell lung carcinoma cell growth through activation of Akt/mammalian target of rapamycin/S6 kinase and inactivation of LKB1/AMP-activated protein kinase signal pathways.Cancer Res. 2006 Jan 1;66(1):315-23. doi: 10.1158/0008-5472.CAN-05-2367. Cancer Res. 2006. PMID: 16397245
-
A complex interplay between Akt, TSC2 and the two mTOR complexes.Biochem Soc Trans. 2009 Feb;37(Pt 1):217-22. doi: 10.1042/BST0370217. Biochem Soc Trans. 2009. PMID: 19143635 Free PMC article. Review.
-
Dysregulation of mTOR activity through LKB1 inactivation.Chin J Cancer. 2013 Aug;32(8):427-33. doi: 10.5732/cjc.013.10086. Epub 2013 May 14. Chin J Cancer. 2013. PMID: 23668926 Free PMC article. Review.
Cited by
-
The Bright Side of Curcumin: A Narrative Review of Its Therapeutic Potential in Cancer Management.Cancers (Basel). 2024 Jul 18;16(14):2580. doi: 10.3390/cancers16142580. Cancers (Basel). 2024. PMID: 39061221 Free PMC article. Review.
-
Effects of Curcumin and Estrogen Receptor Alpha in Luminal Breast Cancer Cells.Diagnostics (Basel). 2024 Aug 16;14(16):1785. doi: 10.3390/diagnostics14161785. Diagnostics (Basel). 2024. PMID: 39202273 Free PMC article.
-
Targeting AMPK for the Alleviation of Pathological Pain.Exp Suppl. 2016;107:257-285. doi: 10.1007/978-3-319-43589-3_11. Exp Suppl. 2016. PMID: 27812984 Free PMC article. Review.
-
Curcumin Analogue C1 Promotes Hex and Gal Recruitment to the Plasma Membrane via mTORC1-Independent TFEB Activation.Int J Mol Sci. 2019 Mar 18;20(6):1363. doi: 10.3390/ijms20061363. Int J Mol Sci. 2019. PMID: 30889901 Free PMC article.
-
Synergistic Interplay between Curcumin and Polyphenol-Rich Foods in the Mediterranean Diet: Therapeutic Prospects for Neurofibromatosis 1 Patients.Nutrients. 2017 Jul 21;9(7):783. doi: 10.3390/nu9070783. Nutrients. 2017. PMID: 28754004 Free PMC article.
References
-
- Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. - PubMed
-
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. - PubMed
-
- Belham C, Wu S, Avruch J. Intracellular signalling: PDK1--a kinase at the hub of things. Curr Biol. 1999;9:R93–6. - PubMed
-
- Toker A, Yoeli-Lerner M. Akt signaling and cancer: surviving but not moving on. Cancer Res. 2006;66:3963–6. - PubMed
-
- Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

