Thematic Review Series: Glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism
- PMID: 18791037
- PMCID: PMC2582367
- DOI: 10.1194/jlr.R800019-JLR200
Thematic Review Series: Glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism
Abstract
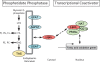
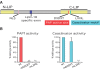
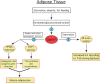
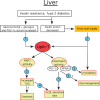
Phosphatidate phosphatase-1 (PAP1) enzymes have a key role in glycerolipid synthesis through the conversion of phosphatidate to diacylglycerol, the immediate precursor of triacylglycerol, phosphatidylcholine, and phosphatidylethanolamine. PAP1 activity in mammals is determined by the lipin family of proteins, lipin-1, lipin-2, and lipin-3, which each have distinct tissue expression patterns and appear to have unique physiological functions. In addition to its role in glycerolipid synthesis, lipin-1 also operates as a transcriptional coactivator, working in collaboration with known nuclear receptors and coactivators to modulate lipid metabolism gene expression. The requirement for different lipin activities in vivo is highlighted by the occurrence of lipodystrophy, insulin resistance, and neuropathy in a lipin-1-deficient mutant mouse strain. In humans, variations in lipin-1 expression levels and gene polymorphisms are associated with insulin sensitivity, metabolic rate, hypertension, and risk for the metabolic syndrome. Furthermore, critical mutations in lipin-2 result in the development of an inflammatory disorder in human patients. A key goal of future studies will be to further elucidate the specific roles and modes of regulation of each of the three lipin proteins in key metabolic processes, including triglyceride and phospholipid synthesis, fatty acid metabolism, and insulin sensitivity.
Figures
References
-
- Langner C. A., E. H. Birkenmeier, O. Ben-Zeev, M. C. Schotz, H. O. Sweet, M. T. Davisson, and J. I. Gordon. 1989. The fatty liver dystrophy (fld) mutation. A new mutant mouse with a developmental abnormality in triglyceride metabolism and associated tissue-specific defects in lipoprotein lipase and hepatic lipase activities. J. Biol. Chem. 264 7994–8003. - PubMed
-
- Reue K., P. Xu, X. P. Wang, and B. G. Slavin. 2000. Adipose tissue deficiency, glucose intolerance, and increased atherosclerosis result from mutation in the mouse fatty liver dystrophy (fld) gene. J. Lipid Res. 41 1067–1076. - PubMed
-
- Garg A. 2004. Acquired and inherited lipodystrophies. N. Engl. J. Med. 350 1220–1234. - PubMed
-
- Péterfy M., J. Phan, P. Xu, and K. Reue. 2001. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat. Genet. 27 121–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

