Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site
- PMID: 18791069
- PMCID: PMC2535569
- DOI: 10.1073/pnas.0804372105
Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site
Abstract
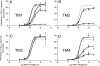
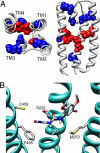
Positive allosteric modulators of alpha7 nicotinic acetylcholine receptors (nAChRs) have attracted considerable interest as potential tools for the treatment of neurological and psychiatric disorders such as Alzheimer's disease and schizophrenia. However, despite the potential therapeutic usefulness of these compounds, little is known about their mechanism of action. Here, we have examined two allosteric potentiators of alpha7 nAChRs (PNU-120596 and LY-2087101). From studies with a series of subunit chimeras, we have identified the transmembrane regions of alpha7 as being critical in facilitating potentiation of agonist-evoked responses. Furthermore, we have identified five transmembrane amino acids that, when mutated, significantly reduce potentiation of alpha7 nAChRs. The amino acids we have identified are located within the alpha-helical transmembrane domains TM1 (S222 and A225), TM2 (M253), and TM4 (F455 and C459). Mutation of either A225 or M253 individually have particularly profound effects, reducing potentiation of EC(20) concentrations of acetylcholine to a tenth of the level seen with wild-type alpha7. Reference to homology models of the alpha7 nAChR, based on the 4A structure of the Torpedo nAChR, indicates that the side chains of all five amino acids point toward an intrasubunit cavity located between the four alpha-helical transmembrane domains. Computer docking simulations predict that the allosteric compounds such as PNU-120596 and LY-2087101 may bind within this intrasubunit cavity, much as neurosteroids and volatile anesthetics are thought to interact with GABA(A) and glycine receptors. Our findings suggest that this is a conserved modulatory allosteric site within neurotransmitter-gated ion channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. Cys-loop receptors: New twists and turns. Trends Neurosci. 2004;27:329–336. - PubMed
-
- Ortells MO, Lunt GG. Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends Neurosci. 1995;18:121–127. - PubMed
-
- Changeux J-P, Edelstein SJ. Allosteric mechanisms of signal transduction. Science. 2005;308:1424–1428. - PubMed
-
- Changeux J-P, Taly A. Nicotinic receptors, allosteric proteins and medicine. Trends Mol Med. 2008;14:93–102. - PubMed
-
- Weiland S, Bertrand D, Leonard S. Neuronal nicotinic acetylcholine receptors: From the gene to the disease. Behav Brain Res. 2000;113:43–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

