Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells
- PMID: 18791452
- PMCID: PMC2637773
- DOI: 10.1097/TP.0b013e31818410a3
Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells
Abstract
Objectives: Intravascular thrombosis remains a major barrier to successful pig-to-primate xenotransplantation. However, the precise factors initiating thrombosis are unknown. In this study, we investigated the contribution of recipient platelets and monocytes.
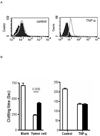
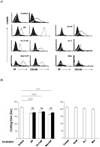
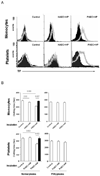
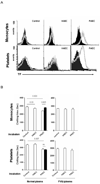
Methods: Primary pig aortic endothelial cells (PAECs) were incubated with combinations of fresh or heat-inactivated human plasma, platelets, or monocytes, after which they were separated and analyzed individually by flow cytometry for tissue factor (TF) expression and for their ability to clot recalcified normal or factor-VII-deficient plasma.
Results: Procoagulant porcine TF was induced in PAECs only by fresh human plasma, and not by heat-inactivated plasma, platelets, or monocytes. In contrast, procoagulant human TF was induced on platelets and monocytes after incubation with PAEC, irrespective of whether the plasma was present or not. In addition, human platelets caused the shedding of procoagulant TF-expressing aggregates from PAEC.
Conclusions: This work defines a cell-based in vitro assay system to address complex interactions among PAECs, human platelets, and monocytes. The induction of procoagulant TF on PAECs by fresh human plasma was most likely dependent on xenoreactive natural antibody and complement present in fresh human plasma. In contrast, the shedding of procoagulant platelet-PAEC aggregates, induced by human platelets, and the induction of procoagulant TF on human platelets and monocytes by PAEC, occurred independently of these factors. These results suggest that different mechanisms may contribute to the initiation of thrombosis after xenotransplantation, some of which may not be influenced by the further manipulation of the immune response against pig xenografts.
Figures


References
-
- Cooper DK, Gollackne B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133. - PubMed
-
- Cooper DK, Dorling A, Pierson RN, 3rd, et al. Alpha1,3-galactosyltransferase gene-knockout pigs for xenotransplantation: where do we go from here? Transplantation. 2007;84(1):1. - PubMed
-
- Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11(1):29. - PubMed
-
- Tseng YL, Kuwaki K, Dor FJ, et al. alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80(10):1493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

