Amelioration of human allograft arterial injury by atorvastatin or simvastatin correlates with reduction of interferon-gamma production by infiltrating T cells
- PMID: 18791454
- PMCID: PMC2650813
- DOI: 10.1097/TP.0b013e318183eefa
Amelioration of human allograft arterial injury by atorvastatin or simvastatin correlates with reduction of interferon-gamma production by infiltrating T cells
Abstract
Background: Graft arteriosclerosis (GA) is an important factor limiting long-term outcomes after organ transplantation. We have used a chimeric humanized mouse system to model this arteriopathy in human vessels, and found that the morphologic and functional changes of experimental GA are interferon (IFN)-gamma dependent. This study evaluated whether 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, described as inhibitors of IFN-gamma production, affect GA in our model.
Methods: C.B.-17 severe combined immunodeficiency-beige mice were transplanted with human artery segments as aortic interposition grafts and inoculated with allogeneic human peripheral blood mononuclear cells (PBMCs) or replication-deficient adenovirus encoding human IFN-gamma. Transplant arteries were analyzed from recipients treated with vehicle vs. atorvastatin or simvastatin at different doses. The effects of statins on T-cell alloresponses to vascular endothelial cells were also investigated in vitro.
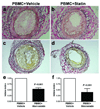
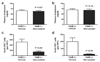
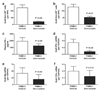
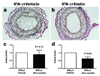
Results: Graft arteriosclerosis-like arteriopathy induced by PBMCs was reduced by atorvastatin at 30 mg/kg/day or simvastatin at 100 mg/kg/day that correlated with decreased graft-infiltrating CD3+ T cells. Circulating IFN-gamma was also reduced, as were graft IFN-gamma and IFN-gamma-inducible chemokine transcripts and graft human leukocyte antigen-DR expression. Graft arteriosclerosis directly induced by human IFN-gamma in the absence of human PBMCs was also reduced by atorvastatin, but only at the highest dose of 100 mg/kg/day. Finally, atorvastatin decreased the clonal expansion and production of interleukin-2, but not IFN-gamma, by human CD4+ T cells in response to allogeneic endothelial cells in coculture.
Conclusions: Our results suggest that a benefit of statin administration in transplantation may include amelioration of GA primarily by inhibiting alloreactive T-cell accumulation and consequent IFN-gamma production and secondarily through suppression of the arterial response to IFN-gamma.
Figures
Similar articles
-
Hydroxymethylglutaryl coenzyme a reductase inhibitors down-regulate chemokines and chemokine receptors in patients with coronary artery disease.J Am Coll Cardiol. 2003 May 7;41(9):1460-7. doi: 10.1016/s0735-1097(03)00263-8. J Am Coll Cardiol. 2003. PMID: 12742282 Clinical Trial.
-
Human allograft arterial injury is ameliorated by sirolimus and cyclosporine and correlates with suppression of interferon-gamma.Transplantation. 2006 Feb 27;81(4):559-66. doi: 10.1097/01.tp.0000198737.12507.19. Transplantation. 2006. PMID: 16495804
-
Treatment of experimental autoimmune uveoretinitis with atorvastatin and lovastatin.Exp Eye Res. 2007 Mar;84(3):569-76. doi: 10.1016/j.exer.2006.11.011. Epub 2007 Jan 17. Exp Eye Res. 2007. PMID: 17208229
-
Comparison of statins in hypertriglyceridemia.Am J Cardiol. 1998 Feb 26;81(4A):66B-69B. doi: 10.1016/s0002-9149(98)00041-1. Am J Cardiol. 1998. PMID: 9526817 Review.
-
Evaluation of atorvastatin and simvastatin for treatment of multiple sclerosis.Expert Rev Neurother. 2007 May;7(5):547-56. doi: 10.1586/14737175.7.5.547. Expert Rev Neurother. 2007. PMID: 17492904 Review.
Cited by
-
Impact of hyperlipidemia on alloimmunity.Curr Opin Organ Transplant. 2017 Feb;22(1):14-21. doi: 10.1097/MOT.0000000000000381. Curr Opin Organ Transplant. 2017. PMID: 27984277 Free PMC article. Review.
-
Randomized clinical trial: atorvastatin versus placebo in patients with acute exacerbation of mild to moderate ulcerative colitis.Indian J Gastroenterol. 2014 Mar;33(2):151-6. doi: 10.1007/s12664-013-0420-4. Epub 2013 Nov 13. Indian J Gastroenterol. 2014. PMID: 24222372 Clinical Trial.
-
Statin intensity and risk for cardiovascular events after heart transplantation.ESC Heart Fail. 2020 Oct;7(5):2074-2081. doi: 10.1002/ehf2.12784. Epub 2020 Jun 24. ESC Heart Fail. 2020. PMID: 32578953 Free PMC article.
-
Peroxisome proliferator-activated receptor-γ agonists prevent in vivo remodeling of human artery induced by alloreactive T cells.Circulation. 2011 Jul 12;124(2):196-205. doi: 10.1161/CIRCULATIONAHA.110.015396. Epub 2011 Jun 20. Circulation. 2011. PMID: 21690493 Free PMC article.
-
Interacting mechanisms in the pathogenesis of cardiac allograft vasculopathy.Arterioscler Thromb Vasc Biol. 2014 Aug;34(8):1609-14. doi: 10.1161/ATVBAHA.114.302818. Epub 2014 Jun 5. Arterioscler Thromb Vasc Biol. 2014. PMID: 24903097 Free PMC article. Review.
References
-
- Libby P, Pober JS. Chronic rejection. Immunity. 2001;14:387. - PubMed
-
- Valantine HA. Cardiac allograft vasculopathy: central role of endothelial injury leading to transplant “atheroma”. Transplantation. 2003;76:891. - PubMed
-
- Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847. - PubMed
-
- Youssef S, Stuve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

