The ability to survive mitosis in the presence of microtubule poisons differs significantly between human nontransformed (RPE-1) and cancer (U2OS, HeLa) cells
- PMID: 18792104
- PMCID: PMC2711993
- DOI: 10.1002/cm.20316
The ability to survive mitosis in the presence of microtubule poisons differs significantly between human nontransformed (RPE-1) and cancer (U2OS, HeLa) cells
Abstract
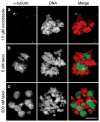
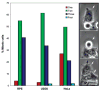
We used live cell imaging to compare the fate of human nontransformed (RPE-1) and cancer (HeLa, U2OS) cells as they entered mitosis in nocodazole or taxol. In the same field, and in either drug, a cell in all lines could die in mitosis, exit mitosis and die within 10 h, or exit mitosis and survive > or =10 h. Relative to RPE-1 cells, significantly fewer HeLa or U2OS cells survived mitosis or remained viable after mitosis: in nocodazole concentrations that inhibit spindle microtubule assembly, or in 500 nM taxol, 30% and 27% of RPE-1 cells, respectively, died in or within 10 h of exiting mitosis while 90% and 49% of U2OS and 78% and 81% of HeLa died. This was even true for clinically relevant taxol concentrations (5 nM) which killed 93% and 46%, respectively, of HeLa and U2OS cells in mitosis or within 10 h of escaping mitosis, compared to 1% of RPE-1 cells. Together these data imply that studies using HeLa or U2OS cells, harvested after a prolonged block in mitosis with nocodazole or taxol, are significantly contaminated with dead or dying cells. We also found that the relationship between the duration of mitosis and survival is drug and cell type specific and that lethality is related to the cell type and drug used to prevent satisfaction of the kinetochore attachment checkpoint. Finally, work with a pan-caspase inhibitor suggests that the primary apoptotic pathway triggered by nocodazole during mitosis in RPE-1 cells is not active in U2OS cells. Cell Motil. Cytoskeleton 2008. (c) 2008 Wiley-Liss, Inc.
Figures



References
-
- Allan LA, Clarke PR. Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol Cell. 2007;26:301–310. - PubMed
-
- Cahill DP, Lengauer C, Yu J, Riggins GJ, Wilson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

