The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88
- PMID: 18792393
- PMCID: PMC7137387
- DOI: 10.1002/hep.22470
The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88
Abstract
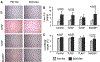
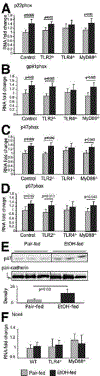
The Toll-like receptor 4 (TLR4) that recognizes endotoxin, a trigger of inflammation in alcoholic liver disease (ALD), activates two signaling pathways utilizing different adapter molecules: the common TLR adapter, myeloid differentiation factor 88 (MyD88), or Toll/interleukin immune-response-domain-containing adaptor inducing interferon (IFN)-beta. The MyD88 pathway induces proinflammatory cytokine activation, a critical mediator of ALD. Here we evaluated the role of MyD88 in alcohol-induced liver injury in wild-type, TLR2-deficient, TLR4-deficient, or MyD88-deficient (knockout [KO]) mice after administration of the Lieber-De-Carli diet (4.5% volume/volume ethanol) or an isocaloric liquid control diet for 5 weeks. Alcohol feeding resulted in a significant increase in serum alanine aminotransferase levels, liver steatosis and triglyceride levels suggesting liver damage in WT, TLR2-KO, and MyD88-KO but not in TLR4-KO mice. Expression of inflammatory mediators (tumor necrosis factor-alpha and interleukin-6) and TLR4 coreceptors (CD14 and MD2) was significantly higher in livers of alcohol-fed WT, TLR2-KO, or MyD88-KO, but not in TLR4-KO mice, compared to controls. Reactive oxygen radicals produced by cytochrome P450 and the nicotinamide adenine dinucleotide phosphate complexes contribute to alcoholic liver damage. Alcohol feeding-induced expression and activation of cytochrome P450 and the nicotinamide adenine dinucleotide phosphate complex were prevented by TLR4-deficiency but not by MyD88-deficiency. Liver expression of interferon regulatory factor 3 (IRF3), a MyD88-independent signaling molecule, was not affected by chronic alcohol treatment in whole livers of WT mice or in any of the KO mice. However, the induction of IRF7, an IRF3-inducible gene, was found in Kupffer cells of alcohol-fed WT mice. Alcohol feeding also induced nuclear factor-kappaB activation in a TLR4-dependent MyD88-independent manner.
Conclusion: While TLR4 deficiency was protective, MyD88 deficiency failed to prevent alcohol-induced liver damage and inflammation. These results suggest that the common TLR adapter, MyD88, is dispensable in TLR4-mediated liver injury in ALD.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures
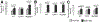
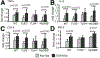


References
-
- Liver Transplantation for Alcoholic Liver Disease. Proceedings of a meeting. Bethesda, Maryland, December 6–7, 1996. Liver Transpl Surg 1997; 3:197–350. - PubMed
-
- Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res 2005; 29(Suppl):166S–171S. - PubMed
-
- Nanji AA. Role of Kupffer cells in alcoholic hepatitis. Alcohol 2002;27: 13–15. - PubMed
-
- Dey A, Cederbaum AI. Alcohol and oxidative liver injury. HEPATOLOGY 2006;43(Suppl 1):S63–S74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
