Regulation of macrophage nitric oxide production by the protein tyrosine phosphatase Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1)
- PMID: 18793215
- PMCID: PMC2678188
- DOI: 10.1111/j.1365-2567.2008.02929.x
Regulation of macrophage nitric oxide production by the protein tyrosine phosphatase Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1)
Abstract
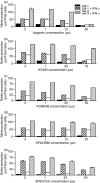
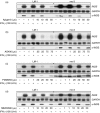
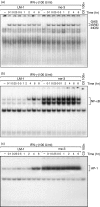
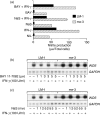
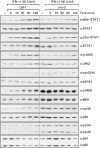
Nitric oxide (NO) is a potent molecule involved in the cytotoxic effects mediated by macrophages (MØ) against microorganisms. We previously reported that Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1)-deficient cells generate a greater amount of NO than wild-type cells in response to interferon-gamma (IFN-gamma). We also reported that the Leishmania-induced MØ SHP-1 activity is needed for the survival of the parasite within phagocytes through the attenuation of NO-dependent and NO-independent mechanisms. In the present study, we investigated the role of SHP-1 in regulating key signalling molecules important in MØ NO generation. Janus tyrosine kinase 2 (JAK2), mitogen-activated extracellular signal-regulated protein kinase kinase (MEK), extracellular signal-regulated kinases 1 and 2 (Erk1/Erk2) mitogen-activated protein kinases, p38 and stress-activated mitogen-activated protein kinases/c-Jun NH(2)-terminal kinase (SAPK/JNK) were examined in immortalized bone marrow-derived MØ (BMDM) from both SHP-1-deficient motheaten mice (me-3) and their respective littermates (LM-1). The results indicated that Erk1/Erk2 and SAPK/JNK are the main kinases regulated by SHP-1 because the absence of SHP-1 caused an increase in their phosphorylation. Moreover, only Apigenin, the specific inhibitor of Erk1/Erk2, was able to block IFN-gamma-induced inducible nitric oxide synthase (iNOS) transcription and translation in me-3 cells. Transcription factor analyses revealed that in the absence of SHP-1, activator protein-1 (AP-1) was activated. The activation of AP-1, and not nuclear factor-kappaB (NF-kappaB) or signal transducer and activator of transcription-1 alpha (STAT-1 alpha), may explain the enhanced NO generation in SHP-1-deficient cells. These observations emphasize the involvement of the MAPKs Erk1/Erk2 and SAPK/JNK in NO generation via AP-1 activation. Collectively, our findings suggest that SHP-1 plays a pivotal role in the negative regulation of signalling events leading to iNOS expression and NO generation. Furthermore, our observations underline the importance of SHP-1-mediated negative regulation in maintaining NO homeostasis and thus preventing the abnormal generation of NO that can be detrimental to the host.
Figures

References
-
- Green SJ, Nacy CA, Meltzer MS. Cytokine-induced synthesis of nitrogen-oxides in macrophages – a protective host response to Leishmania and other intracellular pathogens. J Leukoc Biol. 1991;50:93–103. - PubMed
-
- Liew FY. The role of nitric-oxide in parasitic diseases. Ann Trop Med Parasitol. 1993;87:637–42. - PubMed
-
- Lowenstein CJ, Dinerman JL, Snyder SH. Nitric-oxide – a physiological messenger. Ann Intern Med. 1994;120:227–37. - PubMed
-
- Karupiah G, Xie QW, Buller RML, Nathan C, Duarte C, Macmicking JD. Inhibition of viral replication by interferon-gamma-induced nitric-oxide synthase. Science. 1993;261:1445–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

