Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism
- PMID: 18793399
- PMCID: PMC2553764
- DOI: 10.1186/1742-2094-5-38
Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism
Abstract
Background: Septic encephalopathy is a severe brain dysfunction caused by systemic inflammation in the absence of direct brain infection. Changes in cerebral blood flow, release of inflammatory molecules and metabolic alterations contribute to neuronal dysfunction and cell death.
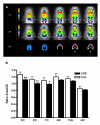
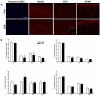
Methods: To investigate the relation of electrophysiological, metabolic and morphological changes caused by SE, we simultaneously assessed systemic circulation, regional cerebral blood flow and cortical electroencephalography in rats exposed to bacterial lipopolysaccharide. Additionally, cerebral glucose uptake, astro- and microglial activation as well as changes of inflammatory gene transcription were examined by small animal PET using [18F]FDG, immunohistochemistry, and real time PCR.
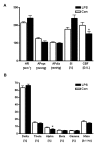
Results: While the systemic hemodynamic did not change significantly, regional cerebral blood flow was decreased in the cortex paralleled by a decrease of alpha activity of the electroencephalography. Cerebral glucose uptake was reduced in all analyzed neocortical areas, but preserved in the caudate nucleus, the hippocampus and the thalamus. Sepsis enhanced the transcription of several pro- and anti-inflammatory cytokines and chemokines including tumor necrosis factor alpha, interleukin-1 beta, transforming growth factor beta, and monocot chemoattractant protein 1 in the cerebrum. Regional analysis of different brain regions revealed an increase in ED1-positive microglia in the cortex, while total and neuronal cell counts decreased in the cortex and the hippocampus.
Conclusion: Together, the present study highlights the complexity of sepsis induced early impairment of neuronal metabolism and activity. Since our model uses techniques that determine parameters relevant to the clinical setting, it might be a useful tool to develop brain specific therapeutic strategies for human septic encephalopathy.
Figures
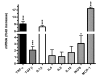
References
-
- Pine RW, Wertz MJ, Lennard ES, Dellinger EP, Carrico CJ, Minshew BH. Determinants of Organ Malfunction Or Death in Patients with Intra-Abdominal Sepsis – A Discriminant-Analysis. Archives of Surgery. 1983;118:242–249. - PubMed
-
- Sprung CL, Peduzzi PN, Shatney CH, Schein RMH, Wilson MF, Sheagren JN, et al. Impact of Encephalopathy on Mortality in the Sepsis Syndrome. Critical Care Medicine. 1990;18:801–806. - PubMed
-
- Wilson JX, Young GB. Progress in clinical neurosciences: Sepsis-associated encephalopathy: Evolving concepts. Canadian Journal of Neurological Sciences. 2003;30:98–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

