Should countries implementing an artemisinin-based combination malaria treatment policy also introduce rapid diagnostic tests?
- PMID: 18793410
- PMCID: PMC2556342
- DOI: 10.1186/1475-2875-7-176
Should countries implementing an artemisinin-based combination malaria treatment policy also introduce rapid diagnostic tests?
Abstract
Background: Within the context of increasing antimalarial costs and or decreasing malaria transmission, the importance of limiting antimalarial treatment to only those confirmed as having malaria parasites becomes paramount. This motivates for this assessment of the cost-effectiveness of routine use of rapid diagnostic tests (RDTs) as an integral part of deploying artemisinin-based combination therapies (ACTs).
Methods: The costs and cost-effectiveness of using RDTs to limit the use of ACTs to those who actually have Plasmodium falciparum parasitaemia in two districts in southern Mozambique were assessed. To evaluate the potential impact of introducing definitive diagnosis using RDTs (costing $0.95), five scenarios were considered, assuming that the use of definitive diagnosis would find that between 25% and 75% of the clinically diagnosed malaria patients are confirmed to be parasitaemic. The base analysis compared two ACTs, artesunate plus sulfadoxine/pyrimethamine (AS+SP) costing $1.77 per adult treatment and artemether-lumefantrine (AL) costing $2.40 per adult treatment, as well as the option of restricting RDT use to only those older than six years. Sensitivity analyses considered lower cost ACTs and RDTs and different population age distributions.
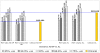
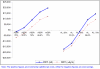
Results: Compared to treating patients on the basis of clinical diagnosis, the use of RDTs in all clinically diagnosed malaria cases results in cost savings only when 29% and 52% or less of all suspected malaria cases test positive for malaria and are treated with AS+SP and AL, respectively. These cut-off points increase to 41.5% (for AS+SP) and to 74% (for AL) when the use of RDTs is restricted to only those older than six years of age. When 25% of clinically diagnosed patients are RDT positive and treated using AL, there are cost savings per malaria positive patient treated of up to $2.12. When more than 29% of clinically diagnosed cases are malaria test positive, the incremental cost per malaria positive patient treated is less than US$1. When relatively less expensive ACTs are introduced (e.g. current WHO preferential price for AL of $1.44 per adult treatment), the RDT price to the healthcare provider should be $0.65 or lower for RDTs to be cost saving in populations with between 30 and 52% of clinically diagnosed malaria cases being malaria test positive.
Conclusion: While the use of RDTs in all suspected cases has been shown to be cost-saving when parasite prevalence among clinically diagnosed malaria cases is low to moderate, findings show that targeting RDTs at the group older than six years and treating children less than six years on the basis of clinical diagnosis is even more cost-saving. In semi-immune populations, young children carry the highest risk of severe malaria and many healthcare providers would find it harder to deny antimalarials to those who test negative in this age group.
Figures




Similar articles
-
Operational response to malaria epidemics: are rapid diagnostic tests cost-effective?Trop Med Int Health. 2006 Apr;11(4):398-408. doi: 10.1111/j.1365-3156.2006.01580.x. Trop Med Int Health. 2006. PMID: 16553923
-
Use of HRP-2-based rapid diagnostic test for Plasmodium falciparum malaria: assessing accuracy and cost-effectiveness in the villages of Dielmo and Ndiop, Senegal.Malar J. 2010 Jun 4;9:153. doi: 10.1186/1475-2875-9-153. Malar J. 2010. PMID: 20525322 Free PMC article.
-
Usefulness of Plasmodium falciparum-specific rapid diagnostic tests for assessment of parasite clearance and detection of recurrent infections after artemisinin-based combination therapy.Malar J. 2013 Oct 1;12:349. doi: 10.1186/1475-2875-12-349. Malar J. 2013. PMID: 24079306 Free PMC article.
-
[Combined antimalarial therapy using artemisinin].Parassitologia. 2004 Jun;46(1-2):85-7. Parassitologia. 2004. PMID: 15305693 Review. Italian.
-
Monitoring antimalarial drug efficacy in the Greater Mekong Subregion: an overview of in vivo results from 2008 to 2010.Southeast Asian J Trop Med Public Health. 2013;44 Suppl 1:201-30; discussion 306-7. Southeast Asian J Trop Med Public Health. 2013. PMID: 24159833 Review.
Cited by
-
Cost-effectiveness of diagnostic for malaria in Extra-Amazon Region, Brazil.Malar J. 2012 Nov 23;11:390. doi: 10.1186/1475-2875-11-390. Malar J. 2012. PMID: 23176717 Free PMC article.
-
New developments in malaria diagnostics: monoclonal antibodies against Plasmodium dihydrofolate reductase-thymidylate synthase, heme detoxification protein and glutamate rich protein.MAbs. 2012 Jan-Feb;4(1):120-6. doi: 10.4161/mabs.4.1.18529. MAbs. 2012. PMID: 22327435 Free PMC article.
-
Exploring provider and community responses to the new malaria diagnostic and treatment regime in Solomon Islands.Malar J. 2011 Jan 10;10:3. doi: 10.1186/1475-2875-10-3. Malar J. 2011. PMID: 21219614 Free PMC article.
-
Cost-effectiveness of malaria microscopy and rapid diagnostic tests versus presumptive diagnosis: implications for malaria control in Uganda.Malar J. 2011 Dec 19;10:372. doi: 10.1186/1475-2875-10-372. Malar J. 2011. PMID: 22182735 Free PMC article.
-
Cost savings with rapid diagnostic tests for malaria in low-transmission areas: evidence from Dar es Salaam, Tanzania.Am J Trop Med Hyg. 2010 Jul;83(1):61-8. doi: 10.4269/ajtmh.2010.09-0632. Am J Trop Med Hyg. 2010. PMID: 20595479 Free PMC article.
References
-
- Goodman CA, Coleman PG, Mills AJ. Economic analysis of malaria control in sub-Saharan Africa. Global Forum for Health Research, Geneva. 2000.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

