Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR
- PMID: 18793453
- PMCID: PMC2551610
- DOI: 10.1186/1471-2180-8-149
Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR
Abstract
Background: To date, only few compounds targeting the AI-2 based quorum sensing (QS) system are known. In the present study, we screened cinnamaldehyde and substituted cinnamaldehydes for their ability to interfere with AI-2 based QS. The mechanism of QS inhibition was elucidated by measuring the effect on bioluminescence in several Vibrio harveyi mutants. We also studied in vitro the ability of these compounds to interfere with biofilm formation, stress response and virulence of Vibrio spp. The compounds were also evaluated in an in vivo assay measuring the reduction of Vibrio harveyi virulence towards Artemia shrimp.
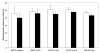
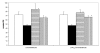
Results: Our results indicate that cinnamaldehyde and several substituted derivatives interfere with AI-2 based QS without inhibiting bacterial growth. The active compounds neither interfered with the bioluminescence system as such, nor with the production of AI-2. Study of the effect in various mutants suggested that the target protein is LuxR. Mobility shift assays revealed a decreased DNA-binding ability of LuxR. The compounds were further shown to (i) inhibit biofilm formation in several Vibrio spp., (ii) result in a reduced ability to survive starvation and antibiotic treatment, (iii) reduce pigment and protease production in Vibrio anguillarum and (iv) protect gnotobiotic Artemia shrimp against virulent Vibrio harveyi BB120.
Conclusion: Cinnamaldehyde and cinnamaldehyde derivatives interfere with AI-2 based QS in various Vibrio spp. by decreasing the DNA-binding ability of LuxR. The use of these compounds resulted in several marked phenotypic changes, including reduced virulence and increased susceptibility to stress. Since inhibitors of AI-2 based quorum sensing are rare, and considering the role of AI-2 in several processes these compounds may be useful leads towards antipathogenic drugs.
Figures



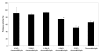


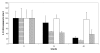

Similar articles
-
Structure-activity relationship of cinnamaldehyde analogs as inhibitors of AI-2 based quorum sensing and their effect on virulence of Vibrio spp.PLoS One. 2011 Jan 13;6(1):e16084. doi: 10.1371/journal.pone.0016084. PLoS One. 2011. PMID: 21249192 Free PMC article.
-
AI-2 quorum-sensing inhibitors affect the starvation response and reduce virulence in several Vibrio species, most likely by interfering with LuxPQ.Microbiology (Reading). 2009 Dec;155(Pt 12):4114-4122. doi: 10.1099/mic.0.032474-0. Epub 2009 Sep 24. Microbiology (Reading). 2009. PMID: 19778962
-
Expression of virulence genes in luminescent and nonluminescent isogenic vibrios and virulence towards gnotobiotic brine shrimp (Artemia franciscana).J Appl Microbiol. 2011 Feb;110(2):399-406. doi: 10.1111/j.1365-2672.2010.04892.x. Epub 2010 Nov 23. J Appl Microbiol. 2011. PMID: 21091862
-
Current advances in Vibrio harveyi quorum sensing as drug discovery targets.Eur J Med Chem. 2020 Dec 1;207:112741. doi: 10.1016/j.ejmech.2020.112741. Epub 2020 Aug 17. Eur J Med Chem. 2020. PMID: 32871343 Review.
-
Quorum Sensing Gene Regulation by LuxR/HapR Master Regulators in Vibrios.J Bacteriol. 2017 Sep 5;199(19):e00105-17. doi: 10.1128/JB.00105-17. Print 2017 Oct 1. J Bacteriol. 2017. PMID: 28484045 Free PMC article. Review.
Cited by
-
Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application.Pathogens. 2015 Mar 23;4(1):137-56. doi: 10.3390/pathogens4010137. Pathogens. 2015. PMID: 25806623 Free PMC article. Review.
-
Antibiofilm Activities of Cinnamaldehyde Analogs against Uropathogenic Escherichia coli and Staphylococcus aureus.Int J Mol Sci. 2022 Jun 29;23(13):7225. doi: 10.3390/ijms23137225. Int J Mol Sci. 2022. PMID: 35806244 Free PMC article.
-
QStatin, a Selective Inhibitor of Quorum Sensing in Vibrio Species.mBio. 2018 Jan 30;9(1):e02262-17. doi: 10.1128/mBio.02262-17. mBio. 2018. PMID: 29382732 Free PMC article.
-
Effect of essential oils on pathogenic bacteria.Pharmaceuticals (Basel). 2013 Nov 25;6(12):1451-74. doi: 10.3390/ph6121451. Pharmaceuticals (Basel). 2013. PMID: 24287491 Free PMC article.
-
Current therapies in treatment and prevention of fracture wound biofilms: why a multifaceted approach is essential for resolving persistent infections.J Bone Jt Infect. 2018 Apr 12;3(2):50-67. doi: 10.7150/jbji.23423. eCollection 2018. J Bone Jt Infect. 2018. PMID: 29761067 Free PMC article. Review.
References
-
- APEC/FAO/NACA/SEMARNAP Report of a joint APEC/FAO/NACA/SEMARNAP ad-hoc expert consultation on trans-boundary aquatic animal pathogen transfer and development of harmonised standards on aquaculture health management. Puerto Vallarta, Jalisco, Mexico. 24–28 july 2000.
-
- Holmström K, Gräslund S, Wahlström A, Poungshompoo S, Bengtsson BE, Kautsky N. Antibiotic use in shrimp farming and implications for environmental impacts and human health. Int J Food Sci Technol. 2003;38:255–266. doi: 10.1046/j.1365-2621.2003.00671.x. - DOI
-
- Tendencia EA, Dela Pena M. Antibiotic resistance of bacteria from shrimp ponds. Aquaculture. 2001;195:193–204. doi: 10.1016/S0044-8486(00)00570-6. - DOI
-
- Karunasagar I, Pai R, Malahti GR, Karunasagar I. Mass mortality of Penaeus monodon larvae due to antibiotic-resistant Vibrio harveyi infection. Aquaculture. 1994;128:203–209. doi: 10.1016/0044-8486(94)90309-3. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

