Thin filament length regulation in striated muscle sarcomeres: pointed-end dynamics go beyond a nebulin ruler
- PMID: 18793739
- PMCID: PMC2650474
- DOI: 10.1016/j.semcdb.2008.08.009
Thin filament length regulation in striated muscle sarcomeres: pointed-end dynamics go beyond a nebulin ruler
Abstract
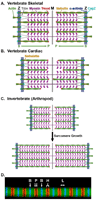
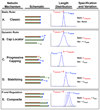
The actin (thin) filaments in striated muscle are highly regulated and precisely specified in length to optimally overlap with the myosin (thick) filaments for efficient myofibril contraction. Here, we review and critically discuss recent evidence for how thin filament lengths are controlled in vertebrate skeletal, vertebrate cardiac, and invertebrate (arthropod) sarcomeres. Regulation of actin polymerization dynamics at the slow-growing (pointed) ends by the capping protein tropomodulin provides a unified explanation for how thin filament lengths are physiologically optimized in all three muscle types. Nebulin, a large protein thought to specify thin filament lengths in vertebrate skeletal muscle through a ruler mechanism, may not control pointed-end actin dynamics directly, but instead may stabilize a large core region of the thin filament. We suggest that this stabilizing function for nebulin modifies the lengths primarily specified by pointed-end actin dynamics to generate uniform filament lengths in vertebrate skeletal muscle. We suggest that nebulette, a small homolog of nebulin, may stabilize a correspondingly shorter core region and allow individual thin filament lengths to vary according to working sarcomere lengths in vertebrate cardiac muscle. We present a unified model for thin filament length regulation where these two mechanisms cooperate to tailor thin filament lengths for specific contractile environments in diverse muscles.
Figures
References
-
- Huxley AF, Niedergerke R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954;173(4412):971–973. - PubMed
-
- Huxley H, Hanson J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954;173(4412):973–976. - PubMed
-
- Huxley HE. Fifty years of muscle and the sliding filament hypothesis. Eur J Biochem. 2004;271(8):1403–1415. - PubMed
-
- Oosawa F. Size distribution of protein polymers. J Theor Biol. 1970;27(1):69–86. - PubMed
-
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112(4):453–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

