Contemporary strategies for the stabilization of peptides in the alpha-helical conformation
- PMID: 18793750
- PMCID: PMC2650020
- DOI: 10.1016/j.cbpa.2008.08.019
Contemporary strategies for the stabilization of peptides in the alpha-helical conformation
Abstract
Herein we review contemporary synthetic and protein design strategies to stabilize the alpha-helical motif in short peptides and miniature proteins. Advances in organometallic catalyst design, specifically for the olefin metathesis reaction, enable the use of hydrocarbon bridges to either crosslink side chains of specific residues or mimic intramolecular hydrogen bonds with carbon-carbon bonds. The resulting hydrocarbon-stapled and hydrogen bond surrogate alpha-helices provide unique synthetic ligands for targeting biomolecules. In the protein design realm, several classes of miniature proteins that display stable helical domains have been engineered and manipulated with powerful in vitro selection technologies to yield libraries of sequences that retain their helical folds. Rational re-design of these scaffolds provide distinctive reagents for the modulation of protein-protein interactions.
Figures
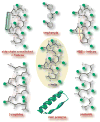


References
-
- Guharoy M, Chakrabarti P. Secondary structure based analysis and classification of biological interfaces: identification of binding motifs in protein-protein interactions. Bioinformatics. 2007;23:1909–1918. - PubMed
-
- Barlow DJ, Thornton JM. Helix Geometry in Proteins. J Mol Biol. 1988;201:601–619. - PubMed
-
- Tyndall JD, Nall T, Fairlie DP. Proteases universally recognize beta strands in their active sites. Chem Rev. 2005;105:973–999. - PubMed
-
- Garner J, Harding MM. Design and synthesis of alpha-helical peptides and mimetics. Org Biomol Chem. 2007;5:3577–3585. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

