Combination therapy with cisplatin and anti-4-1BB: synergistic anticancer effects and amelioration of cisplatin-induced nephrotoxicity
- PMID: 18794112
- PMCID: PMC2551756
- DOI: 10.1158/0008-5472.CAN-08-1365
Combination therapy with cisplatin and anti-4-1BB: synergistic anticancer effects and amelioration of cisplatin-induced nephrotoxicity
Abstract
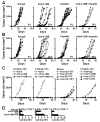
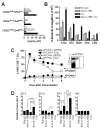
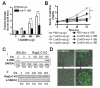
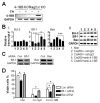
Anti-4-1BB and cisplatin showed synergistic anticancer effects in the CT-26 colon carcinoma model, producing complete regression in >60% of mice with either preventive or therapeutic treatment. The tumor-free mice formed long-lasting CD8(+) T cell-dependent tumor-specific memory. Anti-4-1BB induced rapid repopulation of T and B cells from cisplatin-mediated lymphopenia and differentiation and expansion of IFN-gamma(+)CD11c(+)CD8(+) T cells. Cisplatin facilitated expansion of naïve, effector, and memory CD8(+) T cells; combination therapy produced almost twice as many lymphoid cells as anti-4-1BB alone. Cisplatin increased 4-1BB on antigen-primed T cells and induced 4-1BB de novo on kidney tubular epithelium. Cross-linking of 4-1BB protected the T cells and kidney epithelium from cisplatin-mediated apoptosis by increasing expression of antiapoptotic molecules. Thus, cisplatin-induced 4-1BB provided a mechanism for amelioration of the lymphopenia and nephrotoxicity inherent in cisplatin treatment. We concluded that chemoimmunotherapy with anti-4-1BB and cisplatin is synergistic in tumor killing and prevention of organ-specific toxicity.
Figures
Similar articles
-
Mechanisms involved in synergistic anticancer effects of anti-4-1BB and cyclophosphamide therapy.Mol Cancer Ther. 2009 Feb;8(2):469-78. doi: 10.1158/1535-7163.MCT-08-0993. Epub 2009 Feb 3. Mol Cancer Ther. 2009. PMID: 19190115
-
Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model.Cancer Immunol Res. 2015 Feb;3(2):149-60. doi: 10.1158/2326-6066.CIR-14-0118. Epub 2014 Nov 11. Cancer Immunol Res. 2015. PMID: 25387892
-
Mechanisms involved in synergistic anticancer immunity of anti-4-1BB and anti-CD4 therapy.Cancer Res. 2007 Sep 15;67(18):8891-9. doi: 10.1158/0008-5472.CAN-07-1056. Cancer Res. 2007. PMID: 17875731
-
Exploiting 4-1BB costimulation for enhancing antiviral vaccination.Viral Immunol. 2006 Winter;19(4):593-601. doi: 10.1089/vim.2006.19.593. Viral Immunol. 2006. PMID: 17201654 Review.
-
Dual immunoregulatory pathways of 4-1BB signaling.J Mol Med (Berl). 2006 Sep;84(9):726-36. doi: 10.1007/s00109-006-0072-2. Epub 2006 Aug 5. J Mol Med (Berl). 2006. PMID: 16924475 Review.
Cited by
-
Immune regulatory antibodies: are they the next advance?Cancer J. 2010 Jul-Aug;16(4):311-7. doi: 10.1097/PPO.0b013e3181eb3381. Cancer J. 2010. PMID: 20693841 Free PMC article. Review.
-
Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs.J Transl Med. 2014 Feb 7;12:36. doi: 10.1186/1479-5876-12-36. J Transl Med. 2014. PMID: 24502656 Free PMC article.
-
PCSK9 regulates the efficacy of immune checkpoint therapy in lung cancer.Front Immunol. 2023 Mar 21;14:1142428. doi: 10.3389/fimmu.2023.1142428. eCollection 2023. Front Immunol. 2023. PMID: 37025995 Free PMC article.
-
Effectiveness and safety of PD-1/PD-L1 or CTLA4 inhibitors combined with chemotherapy as a first-line treatment for lung cancer: A meta-analysis.J Thorac Dis. 2018 Dec;10(12):6636-6652. doi: 10.21037/jtd.2018.11.72. J Thorac Dis. 2018. PMID: 30746209 Free PMC article.
-
Immunotherapy with MVA-BN®-HER2 induces HER-2-specific Th1 immunity and alters the intratumoral balance of effector and regulatory T cells.Cancer Immunol Immunother. 2012 Jan;61(1):19-29. doi: 10.1007/s00262-011-1077-4. Epub 2011 Aug 7. Cancer Immunol Immunother. 2012. PMID: 21822917 Free PMC article.
References
-
- Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170(10):4905–13. - PubMed
-
- Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63(15):4490–6. - PubMed
-
- Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34(2):336–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

