The tight junction protein CAR regulates cardiac conduction and cell-cell communication
- PMID: 18794341
- PMCID: PMC2556793
- DOI: 10.1084/jem.20080897
The tight junction protein CAR regulates cardiac conduction and cell-cell communication
Abstract
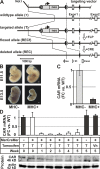
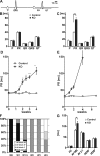
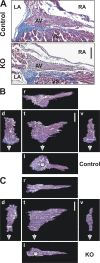
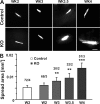
The Coxsackievirus-adenovirus receptor (CAR) is known for its role in virus uptake and as a protein of the tight junction. It is predominantly expressed in the developing brain and heart and reinduced upon cardiac remodeling in heart disease. So far, the physiological functions of CAR in the adult heart are largely unknown. We have generated a heart-specific inducible CAR knockout (KO) and found impaired electrical conduction between atrium and ventricle that increased with progressive loss of CAR. The underlying mechanism relates to the cross talk of tight and gap junctions with altered expression and localization of connexins that affect communication between CAR KO cardiomyocytes. Our results indicate that CAR is not only relevant for virus uptake and cardiac remodeling but also has a previously unknown function in the propagation of excitation from the atrium to the ventricle that could explain the association of arrhythmia and Coxsackievirus infection of the heart.
Figures

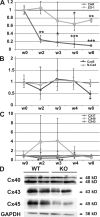

References
-
- Tomaselli, G.F., D.J. Beuckelmann, H.G. Calkins, R.D. Berger, P.D. Kessler, J.H. Lawrence, D. Kass, A.M. Feldman, and E. Marban. 1994. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation. 90:2534–2539. - PubMed
-
- Saffitz, J.E., J.G. Laing, and K.A. Yamada. 2000. Connexin expression and turnover: implications for cardiac excitability. Circ. Res. 86:723–728. - PubMed
-
- Peters, N.S. 2006. Gap junctions: clarifying the complexities of connexins and conduction. Circ. Res. 99:1156–1158. - PubMed
-
- Simon, A.M., D.A. Goodenough, and D.L. Paul. 1998. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr. Biol. 8:295–298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

