Tuberous sclerosis complex proteins control axon formation
- PMID: 18794346
- PMCID: PMC2546692
- DOI: 10.1101/gad.1685008
Tuberous sclerosis complex proteins control axon formation
Abstract
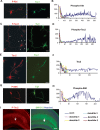
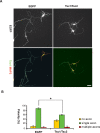
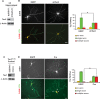
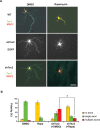
Axon formation is fundamental for brain development and function. TSC1 and TSC2 are two genes, mutations in which cause tuberous sclerosis complex (TSC), a disease characterized by tumor predisposition and neurological abnormalities including epilepsy, mental retardation, and autism. Here we show that Tsc1 and Tsc2 have critical functions in mammalian axon formation and growth. Overexpression of Tsc1/Tsc2 suppresses axon formation, whereas a lack of Tsc1 or Tsc2 function induces ectopic axons in vitro and in the mouse brain. Tsc2 is phosphorylated and inhibited in the axon but not dendrites. Inactivation of Tsc1/Tsc2 promotes axonal growth, at least in part, via up-regulation of neuronal polarity SAD kinase, which is also elevated in cortical tubers of a TSC patient. Our results reveal key roles of TSC1/TSC2 in neuronal polarity, suggest a common pathway regulating polarization/growth in neurons and cell size in other tissues, and have implications for the understanding of the pathogenesis of TSC and associated neurological disorders and for axonal regeneration.
Figures
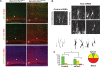
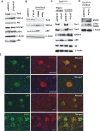
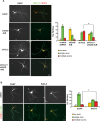
Comment in
-
The Tsc1-Tsc2 complex influences neuronal polarity by modulating TORC1 activity and SAD levels.Genes Dev. 2008 Sep 15;22(18):2447-53. doi: 10.1101/gad.1724108. Genes Dev. 2008. PMID: 18794342 Free PMC article. Review.
References
-
- Arimura N., Kaibuchi K. Neuronal polarity: From extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 2007;8:194–205. - PubMed
-
- Barnes A.P., Lilley B.N., Pan Y.A., Plummer L.J., Powell A.W., Raines A.N., Sanes J.R., Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. - PubMed
-
- Baybis M., Yu J., Lee A., Golden J.A., Weiner H., McKhann G., Aronica E., Crino P.B. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann. Neurol. 2004;56:478–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
