PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex
- PMID: 18794347
- PMCID: PMC2546691
- DOI: 10.1101/gad.1676108
PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex
Abstract
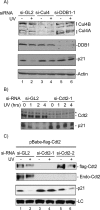
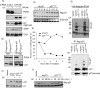
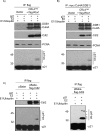
The DNA polymerase delta processivity factor Proliferating Cell Nuclear Antigen (PCNA) promotes the DNA damage-induced degradation of the replication initiation factor Cdt1 via the CRL4(Cdt2) E3 ubiquitin ligase complex. Here we demonstrate that PCNA promotes the ubiquitylation and degradation of the CDK inhibitor p21 in cells irradiated with low dose of ultraviolet (UV) by a similar mechanism. Human cells that are depleted of Cul4, DDB1 (damage-specific DNA-binding protein-1), or the DCAF Cdt2, are deficient in the UV-induced ubiquitylation and degradation of p21. Depletion of mammalian cells of PCNA by siRNA, or mutations in p21 that abrogate PCNA binding, prevent UV-induced p21 ubiquitylation and degradation, indicating that physical binding with PCNA is necessary for the efficient ubiquitylation of p21 via the CRL4(Cdt2) ubiquitin ligase. Cdt2 functions as the substrate recruiting factor for p21 to the rest of the CRL4 ubiquitin ligase complex. The CRL4(Cdt2) E3 ubiquitin ligase ubiquitylates p21 both in vivo and in vitro, and its activity is dependent on the interaction of p21 with PCNA. Finally, we show that the CRL4(Cdt2) and the SCF(Skp2) ubiquitin ligases are redundant with each other in promoting the degradation of p21 during an unperturbed S phase of the cell cycle.
Figures


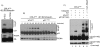


References
-
- Arias E.E., Walter J.C. PCNA functions as a molecular platform to trigger Cdt1 destruction and prevent re-replication. Nat. Cell Biol. 2006;8:84–90. - PubMed
-
- Banks D., Wu M., Higa L.A., Gavrilova N., Quan J., Ye T., Kobayashi R., Sun H., Zhang H. L2DTL/CDT2 and PCNA interact with p53 and regulate p53 polyubiquitination and protein stability through MDM2 and CUL4A/DDB1 complexes. Cell Cycle. 2006;5:1719–1729. - PubMed
-
- Bendjennat M., Boulaire J., Jascur T., Brickner H., Barbier V., Sarasin A., Fotedar A., Fotedar R. UV irradiation triggers ubiquitin-dependent degradation of p21(WAF1) to promote DNA repair. Cell. 2003;114:599–610. - PubMed
-
- Bornstein G., Bloom J., Sitry-Shevah D., Nakayama K., Pagano M., Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 2003;278:25752–25757. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
