Extracellular signal-regulated kinase 2 (ERK2) phosphorylation sites and docking domain on the nuclear pore complex protein Tpr cooperatively regulate ERK2-Tpr interaction
- PMID: 18794356
- PMCID: PMC2573295
- DOI: 10.1128/MCB.00925-08
Extracellular signal-regulated kinase 2 (ERK2) phosphorylation sites and docking domain on the nuclear pore complex protein Tpr cooperatively regulate ERK2-Tpr interaction
Abstract
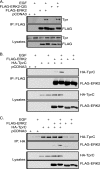
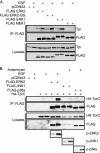
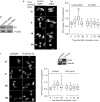
Identifying direct substrates of mitogen-activated protein kinases (MAPKs) and understanding how those substrates are selected is central to understanding how these ubiquitously activated enzymes generate diverse biological responses. In previous work, we identified several new candidate substrates for the MAPK ERK2 (extracellular signal-regulated kinase 2), including the nuclear pore complex protein Tpr (translocated promoter region). In this report, we identify sites on Tpr for ERK2 phosphorylation and binding and demonstrate their functional interaction. ERK2 phosphorylation and dimerization are necessary for ERK2-Tpr binding, and this occurs through a DEF (docking site for ERK2, FXF) domain on Tpr. Surprisingly, the DEF domain and the phosphorylation sites displayed positive cooperativity to promote ERK2 binding to Tpr, in contrast to substrates where phosphorylation reduces binding. Ectopic expression or depletion of Tpr resulted in decreased movement of activated ERK2 from the cytoplasm to the nucleus, implying a role for Tpr in ERK2 translocation. Collectively, the data provide direct evidence that a component of the nuclear pore complex is a bona fide substrate of ERK2 in vivo and that activated ERK2 stably associates with this substrate after phosphorylation, where it could play a continuing role in nuclear pore function. We propose that Tpr is both a substrate and a scaffold for activated ERKs.
Figures







References
-
- Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201110-149. - PubMed
-
- Burack, W. R., and A. S. Shaw. 2005. Live cell imaging of ERK and MEK: simple binding equilibrium explains the regulated nucleocytoplasmic distribution of ERK. J. Biol. Chem. 2803832-3837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
