A novel H19 antisense RNA overexpressed in breast cancer contributes to paternal IGF2 expression
- PMID: 18794369
- PMCID: PMC2573296
- DOI: 10.1128/MCB.02103-07
A novel H19 antisense RNA overexpressed in breast cancer contributes to paternal IGF2 expression
Abstract
The H19/IGFf2 locus belongs to a large imprinted domain located on human chromosome 11p15.5 (homologue to mouse distal chromosome 7). The H19 gene is expressed from the maternal allele, while IGF2 is paternally expressed. Natural antisense transcripts and intergenic transcription have been involved in many aspects of eukaryotic gene expression, including genomic imprinting and RNA interference. However, apart from the identification of some IGF2 antisense transcripts, few data are available on that topic at the H19/IGF2 locus. We identify here a novel transcriptional activity at both the human and the mouse H19/IGF2 imprinted loci. This activity occurs antisense to the H19 gene and has the potential to produce a single 120-kb transcript that we called the 91H RNA. This nuclear and short-lived RNA is not imprinted in mouse but is expressed predominantly from the maternal allele in both mice and humans within the H19 gene region. Moreover, the transcript is stabilized in breast cancer cells and overexpressed in human breast tumors. Finally, knockdown experiments showed that, in humans, 91H, rather than affecting H19 expression, regulates IGF2 expression in trans.
Figures
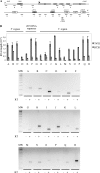

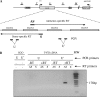
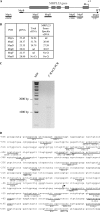

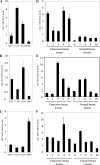



References
-
- Adriaenssens, E., L. Dumont, S. Lottin, D. Bolle, A. Leprêtre, A. Delobelle, F. Bouali, T. Dugimont, J. Coll, and J. J. Curgy. 1998. H19 overexpression in breast adenocarcinoma stromal cells is associated with tumor values and steroid receptor status but independent of p53 and Ki-67 expression. Am. J. Pathol. 1531597-1607. - PMC - PubMed
-
- Ainscough, J. F., T. Koide, M. Tada, S. Barton, and M. A. Surani. 1997. Imprinting of IGF2 and H19 from a 130-kb YAC transgene. Development 1243621-3632. - PubMed
-
- Berteaux, N., S. Lottin, D. Monté, S. Pinte, B. Quatannens, J. Coll, H. Hondermarck, J. J. Curgy, T. Dugimont, and E. Adriaenssens. 2005. H19 mRNA-like noncoding RNA promotes breast cancer cell proliferation through positive control by E2F1. J. Biol. Chem. 28029625-29636. - PubMed
-
- Bestor, T. H. 2000. The DNA methyltransferases of mammals. Hum. Mol. Genet. 92395-2402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
