Phosphorylation switches the general splicing repressor SRp38 to a sequence-specific activator
- PMID: 18794844
- PMCID: PMC2668916
- DOI: 10.1038/nsmb.1485
Phosphorylation switches the general splicing repressor SRp38 to a sequence-specific activator
Abstract
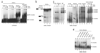
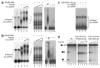
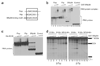
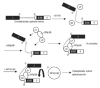
SRp38 is an atypical SR protein that functions as a general splicing repressor when dephosphorylated. We now show that phosphorylated SRp38 functions as a sequence-specific splicing activator. Unlike characterized splicing activators, SRp38 functions in the absence of other SR proteins but requires a cofactor for activity. SRp38 was able to induce formation of splicing complex A in the absence of the cofactor, but this factor was necessary for progression to complexes B and C. Mechanistically, SRp38 strengthens the ability of the U1 and U2 small nuclear ribonucleoproteins to stably recognize the pre-mRNA. Extending these findings, analysis of alternative splicing of pre-mRNA encoding the glutamate receptor B revealed that SRp38 alters its splicing pattern in a sequence-specific manner. Together, our data demonstrate that SRp38, in addition to its role as a splicing repressor, can function as an unusual sequence-specific splicing activator.
Figures



References
-
- Black DL. Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell. 2000;103:367–370. - PubMed
-
- Johnson JM, et al. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–2144. - PubMed
-
- Jurica MS, Moore MJ. Pre-mRNA splicing: awash in a sea of proteins. Mol. Cell. 2003;12:5–14. - PubMed
-
- Smith CW, Valcarcel J. Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem. Sci. 2000;25:381–388. - PubMed
-
- Stamm S. Regulation of alternative splicing by reversible protein phosphorylation. J. Biol. Chem. 2008;283:1223–1227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

