Mycobacterium tuberculosis blocks crosslinking of annexin-1 and apoptotic envelope formation on infected macrophages to maintain virulence
- PMID: 18794848
- PMCID: PMC5351782
- DOI: 10.1038/ni.1654
Mycobacterium tuberculosis blocks crosslinking of annexin-1 and apoptotic envelope formation on infected macrophages to maintain virulence
Abstract
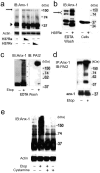
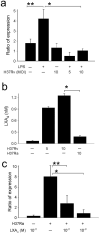
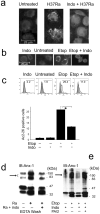
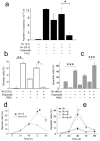
Macrophages infected with attenuated Mycobacterium tuberculosis strain H37Ra become apoptotic, which limits bacterial replication and facilitates antigen presentation. Here we demonstrate that cells infected with H37Ra became apoptotic after the formation of an apoptotic envelope on their surface was complete. This process required exposure of phosphatidylserine on the cell surface, followed by deposition of the phospholipid-binding protein annexin-1 and then transglutaminase-mediated crosslinking of annexin-1 through its amino-terminal domain. In macrophages infected with the virulent strain H37Rv, in contrast, the amino-terminal domain of annexin-1 was removed by proteolysis, thus preventing completion of the apoptotic envelope, which resulted in macrophage death by necrosis. Virulent M. tuberculosis therefore avoids the host defense system by blocking formation of the apoptotic envelope, which leads to macrophage necrosis and dissemination of infection in the lung.
Figures



Comment in
-
Tuberculosis: unsealing the apoptotic envelope.Nat Immunol. 2008 Oct;9(10):1101-2. doi: 10.1038/ni1008-1101. Nat Immunol. 2008. PMID: 18800161 Review.
References
-
- Leemans JC, et al. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J Immunol. 2001;166:4604–4611. - PubMed
-
- Vergne I, Chua J, Singh SB, Deretic V. Cell biology of mycobacterium tuberculosis phagosome. Annu Rev Cell Dev Biol. 2004;20:367–394. - PubMed
-
- Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol. 2000;164:2016–2020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

