Integrins mediate adhesion of medulloblastoma cells to tenascin and activate pathways associated with survival and proliferation
- PMID: 18794852
- PMCID: PMC2679155
- DOI: 10.1038/labinvest.2008.89
Integrins mediate adhesion of medulloblastoma cells to tenascin and activate pathways associated with survival and proliferation
Abstract
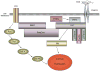
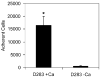
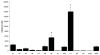
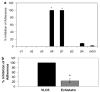
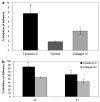
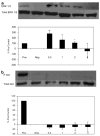
Medulloblastoma spreads by leptomeningeal dissemination rather than by infiltration that characterizes other CNS tumors, eg, gliomas. This study represents an initial attempt to identify both the molecules that mediate medulloblastoma adhesion to leptomeninges and the pathways that are key to survival and proliferation of tumor following adhesion. As a first step in molecule identification, we produced adhesion of D283 medulloblastoma cells to the extracellular matrix (ECM) of H4 glioma cells in vitro. Within this context, D283 cells preferentially expressed the alpha9 and beta1 integrin subunits; antibody and disintegrin blockade of alpha9 and beta1 binding eliminated the adhesion. The H4 ECM was enriched in tenascin, a binding partner for the alpha9beta1 integrin heterodimer. Purified tenascin-C supported D283 cell adhesion. The adhesion was blocked by antibodies to alpha9 and beta1 integrin. In vivo data were similar; immunohistochemistry of primary human medulloblastomas with leptomeningeal extension demonstrated increased expression of alpha9 and beta1 integrins as well as tenascin at the interface of brain and leptomeningeal tumor. These data suggest that tumor-cell expressions of alpha9 and beta1 integrins in combination with extracellular tenascin are necessary for medulloblastoma adhesion to the leptomeninges. As a first step in the identification of pathways that mediate survival and proliferation of tumor following adhesion, we demonstrated that adhesion to H4 ECM was associated with survival and proliferation of D283 cells as well as activation of the MAPK pathway in a growth factor deficient environment. Antibody blockade of alpha9 and beta1 integrin binding that eliminated adhesion also eliminated the in vitro survival benefit. These data suggest that adhesion of medulloblastoma to the meninges is necessary for the survival and proliferation of these tumor cells at the secondary site.
Figures






References
-
- MacDonald TJ, Rood BR, Santi MR, et al. Advances in the diagnosis, molecular genetics, and treatment of pediatric embryonal CNS tumors. Oncologist. 2003;8:174–186. - PubMed
-
- Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–845. - PubMed
-
- Reardon DA, Rich JN, Friedman HS, et al. Recent advances in the treatment of malignant astrocytoma. J Clin Oncol. 2006;24:1253–1265. - PubMed
-
- Mareel M, Leroy A, Bracke M. Cellular and molecular mechanisms of metastasis as applied to carcinomatous meningitis. J Neurooncol. 1998;38:97–102. - PubMed
-
- Grotzer MA, Hogarty MD, Janss AJ, et al. MYC messenger RNA expression predicts survival outcome in childhood primitive neuroectodermal tumor/medulloblastoma. Clin Cancer Res. 2001;7:2425–2433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

