Oridonin confers protection against arsenic-induced toxicity through activation of the Nrf2-mediated defensive response
- PMID: 18795156
- PMCID: PMC2535615
- DOI: 10.1289/ehp.11464
Oridonin confers protection against arsenic-induced toxicity through activation of the Nrf2-mediated defensive response
Abstract
Background: Groundwater contaminated with arsenic imposes a big challenge to human health worldwide. Using natural compounds to subvert the detrimental effects of arsenic represents an attractive strategy. The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) is a critical regulator of the cellular antioxidant response and xenobiotic metabolism. Recently, activation of the Nrf2 signaling pathway has been reported to confer protection against arsenic-induced toxicity in a cell culture model.
Objectives: The goal of the present work was to identify a potent Nrf2 activator from plants as a chemopreventive compound and to demonstrate the efficacy of the compound in battling arsenic-induced toxicity.
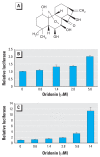
Results: Oridonin activated the Nrf2 signaling pathway at a low subtoxic dose and was able to stabilize Nrf2 by blocking Nrf2 ubiquitination and degradation, leading to accumulation of the Nrf2 protein and activation of the Nrf2-dependent cytoprotective response. Pretreatment of UROtsa cells with 1.4 muM oridonin significantly enhanced the cellular redox capacity, reduced formation of reactive oxygen species (ROS), and improved cell survival after arsenic challenge.
Conclusions: We identified oridonin as representing a novel class of Nrf2 activators and illustrated the mechanism by which the Nrf2 pathway is activated. Furthermore, we demonstrated the feasibility of using natural compounds targeting Nrf2 as a therapeutic approach to protect humans from various environmental insults that may occur daily.
Keywords: ARE; Keap1; Nrf2; antioxidant responsive element; antitumor; arsenic; chemoprevention; diterpenoid; oridonin; oxidative stress; rubescensin.
Figures



Similar articles
-
Arsenic-mediated activation of the Nrf2-Keap1 antioxidant pathway.J Biochem Mol Toxicol. 2013 Feb;27(2):99-105. doi: 10.1002/jbt.21463. Epub 2012 Nov 27. J Biochem Mol Toxicol. 2013. PMID: 23188707 Free PMC article. Review.
-
Oridonin exerts anticancer effect on osteosarcoma by activating PPAR-γ and inhibiting Nrf2 pathway.Cell Death Dis. 2018 Jan 11;9(1):15. doi: 10.1038/s41419-017-0031-6. Cell Death Dis. 2018. PMID: 29323103 Free PMC article.
-
Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents.Methods Mol Biol. 2010;647:37-74. doi: 10.1007/978-1-60761-738-9_3. Methods Mol Biol. 2010. PMID: 20694660 Review.
-
Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways.Cell Commun Signal. 2019 Jun 11;17(1):62. doi: 10.1186/s12964-019-0366-y. Cell Commun Signal. 2019. PMID: 31186013 Free PMC article.
-
Non-covalent NRF2 Activation Confers Greater Cellular Protection than Covalent Activation.Cell Chem Biol. 2019 Oct 17;26(10):1427-1435.e5. doi: 10.1016/j.chembiol.2019.07.011. Epub 2019 Aug 8. Cell Chem Biol. 2019. PMID: 31402317 Free PMC article.
Cited by
-
Curcumin protects human keratinocytes against inorganic arsenite-induced acute cytotoxicity through an NRF2-dependent mechanism.Oxid Med Cell Longev. 2013;2013:412576. doi: 10.1155/2013/412576. Epub 2013 Apr 21. Oxid Med Cell Longev. 2013. PMID: 23710286 Free PMC article.
-
Carbonyl stress in aging process: role of vitamins and phytochemicals as redox regulators.Aging Dis. 2013 Oct 1;4(5):276-94. doi: 10.14336/AD.2013.0400276. Aging Dis. 2013. PMID: 24124633 Free PMC article. Review.
-
Withania somnifera Extract Protects Model Neurons from In Vitro Traumatic Injury.Cell Transplant. 2017 Jul;26(7):1193-1201. doi: 10.1177/0963689717714320. Cell Transplant. 2017. PMID: 28933215 Free PMC article.
-
Defenses against Pro-oxidant Forces - Maintenance of Cellular and Genomic Integrity and Longevity.Radiat Res. 2018 Oct;190(4):331-349. doi: 10.1667/RR15101.1. Epub 2018 Jul 24. Radiat Res. 2018. PMID: 30040046 Free PMC article. Review.
-
Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62-dependent manner.Mol Cell Biol. 2013 Jun;33(12):2436-46. doi: 10.1128/MCB.01748-12. Epub 2013 Apr 15. Mol Cell Biol. 2013. PMID: 23589329 Free PMC article.
References
-
- Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol. 2001;173(3):154–160. - PubMed
-
- Aono J, Yanagawa T, Itoh K, Li B, Yoshida H, Kumagai Y, et al. Activation of Nrf2 and accumulation of ubiquitinated A170 by arsenic in osteoblasts. Biochem Biophys Res Commun. 2003;305(2):271–277. - PubMed
-
- Chan JY, Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim Biophys Acta. 2000;1517(1):19–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

