In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens
- PMID: 18795164
- PMCID: PMC2535623
- DOI: 10.1289/ehp.11200
In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens
Abstract
Background: Concerns have been raised about the biological and toxicologic effects of the antimicrobials triclocarban (TCC) and triclosan (TCS) in personal care products. Few studies have evaluated their biological activities in mammalian cells to assess their potential for adverse effects.
Objectives: In this study, we assessed the activity of TCC, its analogs, and TCS in in vitro nuclear-receptor-responsive and calcium signaling bioassays.
Materials and methods: We determined the biological activities of the compounds in in vitro, cell-based, and nuclear-receptor-responsive bioassays for receptors for aryl hydrocarbon (AhR), estrogen (ER), androgen (AR), and ryanodine (RyR1).
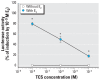
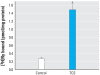
Results: Some carbanilide compounds, including TCC (1-10 muM), enhanced estradiol (E(2))-dependent or testosterone-dependent activation of ER- and AR-responsive gene expression up to 2.5-fold but exhibited little or no agonistic activity alone. Some carbanilides and TCS exhibited weak agonistic and/or antagonistic activity in the AhR-responsive bioassay. TCS exhibited antagonistic activity in both ER- and AR-responsive bioassays. TCS (0.1-10 muM) significantly enhanced the binding of [(3)H]ryanodine to RyR1 and caused elevation of resting cytosolic [Ca(2+)] in primary skeletal myotubes, but carbanilides had no effect.
Conclusions: Carbanilides, including TCC, enhanced hormone-dependent induction of ER- and AR-dependent gene expression but had little agonist activity, suggesting a new mechanism of action of endocrine-disrupting compounds. TCS, structurally similar to noncoplanar ortho-substituted poly-chlorinated biphenyls, exhibited weak AhR activity but interacted with RyR1 and stimulated Ca(2+) mobilization. These observations have potential implications for human and animal health. Further investigations are needed into the biological and toxicologic effects of TCC, its analogs, and TCS.
Keywords: androgen receptor; antimicrobial; aryl hydrocarbon receptor; bioactivity; carbanilide analog; estrogen receptor; ryanodine receptor; sensitization; signal amplification; triclocarban; triclosan.
Figures







References
-
- Adolfsson-Erici M, Pettersson M, Parkkonen J, Sturve J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere. 2002;46:1485–1489. - PubMed
-
- Allmyr M, Adolfsson-Erici M, McLachlan MS, Sandborgh-Englund G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci Total Environ. 2006;372:87–93. - PubMed
-
- Chang CY, McDonnell DP. Androgen receptor-cofactor interactions as targets for new drug discovery. Trends Pharmacol Sci. 2005;26:225–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

