Inhaled asbestos exacerbates atherosclerosis in apolipoprotein E-deficient mice via CD4+ T cells
- PMID: 18795166
- PMCID: PMC2535625
- DOI: 10.1289/ehp.11172
Inhaled asbestos exacerbates atherosclerosis in apolipoprotein E-deficient mice via CD4+ T cells
Abstract
Background: Associations between air pollution and morbidity/mortality from cardiovascular disease are recognized in epidemiologic and clinical studies, but the mechanisms by which inhaled fibers or particles mediate the exacerbation of atherosclerosis are unclear.
Objective and methods: To determine whether lung inflammation after inhalation of a well-characterized pathogenic particulate, chrysotile asbestos, is directly linked to exacerbation of atherosclerosis and the mechanisms involved, we exposed apolipoprotein E-deficient (ApoE(-/-)) mice and ApoE(-/-) mice crossed with CD4(-/-) mice to ambient air, NIEHS (National Institute of Environmental Health Sciences) reference sample of chrysotile asbestos, or fine titanium dioxide (TiO(2)), a nonpathogenic control particle, for 3, 9, or 30 days.
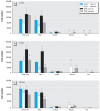
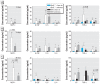
Results: ApoE(-/-) mice exposed to inhaled asbestos fibers had approximately 3-fold larger atherosclerotic lesions than did TiO(2)-exposed ApoE(-/-) mice or asbestos-exposed ApoE(-/-)/CD4(-/-) double-knockout (DKO) mice. Lung inflammation and the magnitude of lung fibrosis assessed histologically were similar in asbestos-exposed ApoE(-/-) and DKO mice. Monocyte chemoattractant protein-1 (MCP-1) levels were increased in bronchoalveolar lavage fluid and plasma, and plasma concentrations correlated with lesion size (p < 0.04) in asbestos-exposed ApoE(-/-) mice. At 9 days, activator protein-1 (AP-1) and nuclear factor-kappaB (NF-kappaB), transcription factors linked to inflammation and found in the promoter region of the MCP-1 gene, were increased in aortas of asbestos-exposed ApoE(-/-) but not DKO mice.
Conclusion: Our findings show that the degree of lung inflammation and fibrosis does not correlate directly with cardiovascular effects of inhaled asbestos fibers and support a critical role of CD4(+) T cells in linking fiber-induced pulmonary signaling to consequent activation of AP-1- and NF-kappaB-regulated genes in atherogenesis.
Keywords: AP-1; CD4+ T-cells; MCP-1; NF-κB; atherosclerosis; chrysotile asbestos; fibrosis; inflammation; knockout mice; lung.
Figures
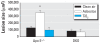




References
-
- BéruBé KA, Quinlan TR, Moulton G, Hemenway D, O’Shaughnessy P, Vacek P, et al. Comparative proliferative and histopathologic changes in rat lungs after inhalation of chrysotile or crocidolite asbestos. Toxicol Appl Pharmacol. 1996;137:67–74. - PubMed
-
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. - PubMed
-
- CDC (Centers for Disease Control and Prevention) Potential exposures to airborne and settled surface dust in residential areas of lower Manhattan following the collapse of the World Trade Center—New York City, November 4-December 11, 2001. Morbid Mortal Wkly Rep. 2003;52:131–136. - PubMed
-
- Craighead JE. Asbestos-associated dieases: report of the Pneumoconiosis Committee of the College of American Pathologists and the National Institute for Occupational Safety and Health. Arch Pathol Lab Med. 1982;106:543–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
