The peripheral neuropathy-linked Trembler and Trembler-J mutant forms of peripheral myelin protein 22 are folding-destabilized
- PMID: 18795802
- PMCID: PMC2566783
- DOI: 10.1021/bi801157p
The peripheral neuropathy-linked Trembler and Trembler-J mutant forms of peripheral myelin protein 22 are folding-destabilized
Abstract
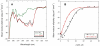
Dominant mutations in the tetraspan membrane protein peripheral myelin protein 22 (PMP22) are known to result in peripheral neuropathies such as Charcot-Marie-Tooth type 1A (CMT1A) disease via mechanisms that appear to be closely linked to misfolding of PMP22 in the membrane of the endoplasmic reticulum (ER). To characterize the molecular defects in PMP22, we examined the structure and stability of two human disease mutant forms of PMP22 that are also the basis for mouse models of peripheral neuropathies: G150D ( Trembler phenotype) and L16P ( Trembler-J phenotype). Circular dichroism and NMR spectroscopic studies indicated that, when folded, the three-dimensional structures of these disease-linked mutants are similar to that of the folded wild-type protein. However, the folded forms of the mutants were observed to be destabilized relative to the wild-type protein, with the L16P mutant being particularly unstable. The rate of refolding from an unfolded state was observed to be very slow for the wild-type protein, and no refolding was observed for either mutant. These results lead to the hypothesis that ER quality control recognizes the G150D and L16P mutant forms of PMP22 as defective through mechanisms closely related to their conformational instability and/or slow folding. It was also seen that wild-type PMP22 binds Zn(II) and Cu(II) with micromolar affinity, a property that may be important to the stability and function of this protein. Zn(II) was able to rescue the stability defect of the Tr mutant.
Figures
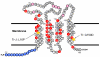





References
-
- Bronstein JM. Function of tetraspan proteins in the myelin sheath. Curr. Opin. Neurobiol. 2000;10:552–557. - PubMed
-
- Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U. Hypermyelination and demyelinating peripheral neuropathy in Pmp22-deficient mice. Nat. Genet. 1995;11:274–280. - PubMed
-
- Carenini S, Neuberg D, Schachner M, Suter U, Martini R. Localization and functional roles of PMP22 in peripheral nerves of P0-deficient mice. Glia. 1999;28:256–264. - PubMed
-
- Taylor V, Zgraggen C, Naef R, Suter U. Membrane topology of peripheral myelin protein 22. J. Neurosci. Res. 2000;62:15–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

