Tissue-type plasminogen activator requires a co-receptor to enhance NMDA receptor function
- PMID: 18796005
- PMCID: PMC3198853
- DOI: 10.1111/j.1471-4159.2008.05687.x
Tissue-type plasminogen activator requires a co-receptor to enhance NMDA receptor function
Abstract
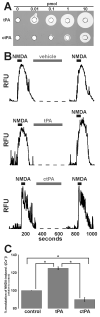
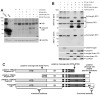
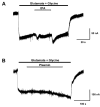
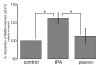
Glutamate is the main excitatory neurotransmitter of the CNS. Tissue-type plasminogen activator (tPA) is recognized as a modulator of glutamatergic neurotransmission. This attribute is exemplified by its ability to potentiate calcium signaling following activation of the glutamate-binding NMDA receptor (NMDAR). It has been hypothesized that tPA can directly cleave the NR1 subunit of the NMDAR and thereby potentiate NMDA-induced calcium influx. In contrast, here we show that this increase in NMDAR signaling requires tPA to be proteolytically active, but does not involve cleavage of the NR1 subunit or plasminogen. Rather, we demonstrate that enhancement of NMDAR function by tPA is mediated by a member of the low-density lipoprotein receptor (LDLR) family. Hence, this study proposes a novel functional relationship between tPA, the NMDAR, a LDLR and an unknown substrate which we suspect to be a serpin. Interestingly, whilst tPA alone failed to cleave NR1, cell-surface NMDARs did serve as an efficient and discrete proteolytic target for plasmin. Hence, plasmin and tPA can affect the NMDAR via distinct avenues. Altogether, we find that plasmin directly proteolyses the NMDAR whilst tPA functions as an indirect modulator of NMDA-induced events via LDLR engagement.
Figures


Similar articles
-
The serine protease plasmin cleaves the amino-terminal domain of the NR2A subunit to relieve zinc inhibition of the N-methyl-D-aspartate receptors.J Biol Chem. 2009 May 8;284(19):12862-73. doi: 10.1074/jbc.M805123200. Epub 2009 Feb 24. J Biol Chem. 2009. PMID: 19240037 Free PMC article.
-
Tissue-type plasminogen activator controls neuronal death by raising surface dynamics of extrasynaptic NMDA receptors.Cell Death Dis. 2016 Nov 10;7(11):e2466. doi: 10.1038/cddis.2016.279. Cell Death Dis. 2016. PMID: 27831563 Free PMC article.
-
Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits.Nature. 2002 Feb 14;415(6873):793-8. doi: 10.1038/nature715. Epub 2002 Jan 30. Nature. 2002. PMID: 11823786
-
Reprint of: Fibrinolytic and Non-fibrinolytic Roles of Tissue-type Plasminogen Activator in the Ischemic Brain.Neuroscience. 2024 Jul 9;550:21-29. doi: 10.1016/j.neuroscience.2024.05.040. Epub 2024 Jul 2. Neuroscience. 2024. PMID: 38964373 Review.
-
Tissue plasminogen activator in the amygdala: a new role for an old protease.J Physiol Pharmacol. 2008 Dec;59 Suppl 8:135-46. J Physiol Pharmacol. 2008. PMID: 19258671 Review.
Cited by
-
Tissue-type plasminogen activator has a neuroprotective effect in the ischemic brain mediated by neuronal TNF-α.J Cereb Blood Flow Metab. 2012 Jan;32(1):57-69. doi: 10.1038/jcbfm.2011.106. Epub 2011 Jul 27. J Cereb Blood Flow Metab. 2012. PMID: 21792242 Free PMC article.
-
Impacts of tissue-type plasminogen activator (tPA) on neuronal survival.Front Cell Neurosci. 2015 Oct 16;9:415. doi: 10.3389/fncel.2015.00415. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26528141 Free PMC article. Review.
-
Stroke patients develop antibodies that react with components of N-methyl-D-aspartate receptor subunit 1 in proportion to lesion size.Stroke. 2013 Aug;44(8):2212-9. doi: 10.1161/STROKEAHA.113.001235. Epub 2013 May 30. Stroke. 2013. PMID: 23723305 Free PMC article.
-
The serine protease plasmin cleaves the amino-terminal domain of the NR2A subunit to relieve zinc inhibition of the N-methyl-D-aspartate receptors.J Biol Chem. 2009 May 8;284(19):12862-73. doi: 10.1074/jbc.M805123200. Epub 2009 Feb 24. J Biol Chem. 2009. PMID: 19240037 Free PMC article.
-
Proteolytic cleavage of proBDNF into mature BDNF in the basolateral amygdala is necessary for defeat-induced social avoidance.Learn Mem. 2016 Mar 15;23(4):156-60. doi: 10.1101/lm.040253.115. Print 2016 Apr. Learn Mem. 2016. PMID: 26980783 Free PMC article.
References
-
- Backes BJ, Harris JL, Leonetti F, Craik CS, Ellman JA. Synthesis of positional-scanning libraries of fluorogenic peptide substrates to define the extended substrate specificity of plasmin and thrombin. Nat Biotechnol. 2000;18:187–193. - PubMed
-
- Benchenane K, Castel H, Boulouard M, et al. Anti-NR1 N-terminal-domain vaccination unmasks the crucial action of tPA on NMDA-receptor-mediated toxicity and spatial memory. J Cell Sci. 2007;120:578–585. - PubMed
-
- Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. - PubMed
-
- Clark RJ, Fischer H, Nevin ST, Adams DJ, Craik DJ. The synthesis, structural characterization, and receptor specificity of the alpha-conotoxin Vc1.1. J Biol Chem. 2006;281:23254–23263. - PubMed
-
- Fernandez-Monreal M, Lopez-Atalaya JP, Benchenane K, et al. Arginine 260 of the amino-terminal domain of NR1 subunit is critical for tissue-type plasminogen activator-mediated enhancement of N-methyl-D-aspartate receptor signaling. J Biol Chem. 2004a;279:50850–50856. - PubMed

