Red fluorescence in reef fish: a novel signalling mechanism?
- PMID: 18796150
- PMCID: PMC2567963
- DOI: 10.1186/1472-6785-8-16
Red fluorescence in reef fish: a novel signalling mechanism?
Abstract
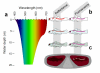
Background: At depths below 10 m, reefs are dominated by blue-green light because seawater selectively absorbs the longer, 'red' wavelengths beyond 600 nm from the downwelling sunlight. Consequently, the visual pigments of many reef fish are matched to shorter wavelengths, which are transmitted better by water. Combining the typically poor long-wavelength sensitivity of fish eyes with the presumed lack of ambient red light, red light is currently considered irrelevant for reef fish. However, previous studies ignore the fact that several marine organisms, including deep sea fish, produce their own red luminescence and are capable of seeing it.
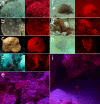
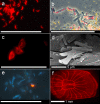
Results: We here report that at least 32 reef fishes from 16 genera and 5 families show pronounced red fluorescence under natural, daytime conditions at depths where downwelling red light is virtually absent. Fluorescence was confirmed by extensive spectrometry in the laboratory. In most cases peak emission was around 600 nm and fluorescence was associated with guanine crystals, which thus far were known for their light reflecting properties only. Our data indicate that red fluorescence may function in a context of intraspecific communication. Fluorescence patterns were typically associated with the eyes or the head, varying substantially even between species of the same genus. Moreover red fluorescence was particularly strong in fins that are involved in intraspecific signalling. Finally, microspectrometry in one fluorescent goby, Eviota pellucida, showed a long-wave sensitivity that overlapped with its own red fluorescence, indicating that this species is capable of seeing its own fluorescence.
Conclusion: We show that red fluorescence is widespread among marine fishes. Many features indicate that it is used as a private communication mechanism in small, benthic, pair- or group-living fishes. Many of these species show quite cryptic colouration in other parts of the visible spectrum. High inter-specific variation in red fluorescence and its association with structures used in intra-specific signalling further corroborate this view. Our findings challenge the notion that red light is of no importance to marine fish, calling for a reassessment of its role in fish visual ecology in subsurface marine environments.
Figures





Similar articles
-
Red fluorescence increases with depth in reef fishes, supporting a visual function, not UV protection.Proc Biol Sci. 2014 Sep 7;281(1790):20141211. doi: 10.1098/rspb.2014.1211. Proc Biol Sci. 2014. PMID: 25030989 Free PMC article.
-
Long-wave sensitivity in deep-sea stomiid dragonfish with far-red bioluminescence: evidence for a dietary origin of the chlorophyll-derived retinal photosensitizer of Malacosteus niger.Philos Trans R Soc Lond B Biol Sci. 2000 Sep 29;355(1401):1269-72. doi: 10.1098/rstb.2000.0681. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11079412 Free PMC article. Review.
-
Hydroids (Cnidaria, Hydrozoa) from Mauritanian Coral Mounds.Zootaxa. 2020 Nov 16;4878(3):zootaxa.4878.3.2. doi: 10.11646/zootaxa.4878.3.2. Zootaxa. 2020. PMID: 33311142
-
The eyes of deep-sea fish. I: Lens pigmentation, tapeta and visual pigments.Prog Retin Eye Res. 1998 Oct;17(4):597-636. doi: 10.1016/s1350-9462(98)00002-0. Prog Retin Eye Res. 1998. PMID: 9777651 Review.
-
Spectral Diversity and Regulation of Coral Fluorescence in a Mesophotic Reef Habitat in the Red Sea.PLoS One. 2015 Jun 24;10(6):e0128697. doi: 10.1371/journal.pone.0128697. eCollection 2015. PLoS One. 2015. PMID: 26107282 Free PMC article.
Cited by
-
Sea as a color palette: the ecology and evolution of fluorescence.Zoological Lett. 2020 Jun 10;6:9. doi: 10.1186/s40851-020-00161-9. eCollection 2020. Zoological Lett. 2020. PMID: 32537244 Free PMC article. Review.
-
Shining in the dark: First record of biofluorescence in the seahorse Hippocampus reidi.PLoS One. 2019 Aug 8;14(8):e0220561. doi: 10.1371/journal.pone.0220561. eCollection 2019. PLoS One. 2019. PMID: 31393893 Free PMC article.
-
Fluorescence as a means of colour signal enhancement.Philos Trans R Soc Lond B Biol Sci. 2017 Jul 5;372(1724):20160335. doi: 10.1098/rstb.2016.0335. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28533452 Free PMC article. Review.
-
Multiple Genetic Mechanisms Contribute to Visual Sensitivity Variation in the Labridae.Mol Biol Evol. 2016 Jan;33(1):201-15. doi: 10.1093/molbev/msv213. Epub 2015 Oct 12. Mol Biol Evol. 2016. PMID: 26464127 Free PMC article.
-
Repeated and widespread evolution of biofluorescence in marine fishes.Nat Commun. 2025 May 24;16(1):4826. doi: 10.1038/s41467-025-59843-7. Nat Commun. 2025. PMID: 40413187 Free PMC article.
References
-
- Marshall NJ, Jennings K, McFarland WN, Loew ER, Losey GS. Visual biology of Hawaiian coral reef fishes. III. Environmental light and an integrated approach to the ecology of reef fish vision. Copeia. 2003;2003:467–480. doi: 10.1643/01-056. - DOI
-
- Loew ER, Zhang H. Propagation of visual signals in the aquatic environment: An interactive windows-based model. In: Ladich F, Collin SP, Moller P, Kapoor BG, editor. Communication in Fishes. Vol. 2. Enfield (NH): Science Publishers; 2006. pp. 281–302.
-
- Losey GS, McFarland WN, Loew ER, Zamzow JP, Nelson PA, Marshall NJ. Visual biology of Hawaiian coral reef fishes. I. Ocular transmission and visual pigments. Copeia. 2003;2003:433–454. doi: 10.1643/01-053. - DOI
-
- Siebeck UE, Losey GS, Marshall J. UV Communication in Fish. In: Ladich F, Collin SP, Moller P, Kapoor BG, editor. Communication in Fishes. Vol. 2. Enfield (NH): Science Publishers; 2006. pp. 423–455.
-
- Marshall J, Vorobyev M, Siebeck UE. What does a reef fish see when it sees a reef fish? Eating 'Nemo'©. In: Ladich F, Collin SP, Moller P, Kapoor BG, editor. Communication in Fishes. Vol. 2. Enfield (NH): Science Publishers; 2006. pp. 393–422.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

