Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial
- PMID: 18796160
- PMCID: PMC2565674
- DOI: 10.1186/1471-2377-8-33
Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial
Abstract
Background: Recent consensus guidelines recommend pregabalin as a first-tier treatment for painful diabetic peripheral neuropathy (DPN). We evaluated the efficacy of pregabalin 600 mg/d (300 mg dosed BID) versus placebo for relieving DPN-associated neuropathic pain, and assessed its safety using objective measures of nerve conduction (NC).
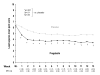
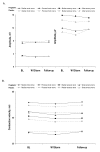
Methods: In this randomized, double-blind, placebo-controlled trial, the primary efficacy measure was endpoint mean pain score (MPS) from daily pain diaries (11-point scale). NC velocity and sensory and motor amplitudes were assessed at baseline, endpoint, and end of follow-up (2 weeks post-treatment). At each timepoint, the median-motor, median-sensory, ulnar-sensory, and peroneal-motor nerves were evaluated. Secondary efficacy measures included weekly MPS and proportion of responders (patients achieving >or=50% reduction in MPS from baseline to endpoint). After 1-weeks' dosage escalation, pregabalin-treated patients received 300 mg BID for 12 weeks.
Results: Eighty-two patients received pregabalin and 85 placebo. Mean durations were 10 years for diabetes and approximately 5 years for painful DPN. Pregabalin-treated patients had lower MPS than controls (mean difference, -1.28; p <.001). For all four nerves, 95% CIs for median differences in amplitude and velocity from baseline to endpoint and baseline to follow-up included 0 (ie, no significant difference vs. placebo). Significant pain improvement among pregabalin-treated patients was evident at week 1 and sustained at every weekly timepoint. More pregabalin-treated patients (49%) than controls (23%) were responders (p <.001).
Conclusion: Pregabalin 600 mg/d (300 mg BID) effectively reduced pain, was well tolerated, and had no statistically significant or clinically meaningful effect on NC in patients with painful DPN.
Trial registration: ClinicalTrials.gov NCT00159679.
Figures


References
-
- Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350–354. - PubMed
-
- Dyck PJ. Pathology and pathophysiology – human and experimental. In: Dyck PJ, Thomas PK, Asbury AK, Winegrad AI, Porte D Jr, editor. Diabetic Neuropathy. Philadelphia: WB Saunders and Company; 1987. pp. 223–236.
-
- Yagihashi S. Pathology and pathogenetic mechanisms of diabetic neuropathy. Diabetes Metab Rev. 1995;11:193–225. - PubMed
-
- Boulton AJM, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27:1458–1486. - PubMed
-
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

